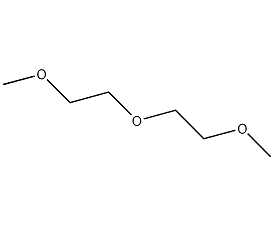
Structural formula
| Business number | 034R |
|---|---|
| Molecular formula | C6H14O3 |
| Molecular weight | 134.17 |
| label |
diglyme, Bis(2-methoxyethyl) ether, Diethylene glycol dimethyl ether, 2-methoxyethyl ether, Dimethylcarbitol, 2-Methoxyethyl ether, Bis(2-methoxyethyl)ether, Dimethyl carbitol, Diglyme, Aprotic polar solvent, linear compound |
Numbering system
CAS number:111-96-6
MDL number:MFCD00008503
EINECS number:203-924-4
RTECS number:KN3339000
BRN number:1736101
PubChem number:24856987
Physical property data
1. Properties: Colorless and transparent liquid with a slight ether smell.
2. Relative density (g/mL, 25/4℃): 0.9449
3. Relative vapor density (g/mL, air=1): 4.6
4. Melting point (ºC): -68
5. Boiling point (ºC, 101.3kPa): 162
6. Refractive index (20ºC): 1.4097
7. Refractive index (25ºC): 1.4043
8. Flash point (ºC): 70
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): 188
11. Vapor pressure (mmHg, 20ºC): 3
12. Saturated vapor pressure (kPa, 25ºC ): 0.45
13. Heat of evaporation (KJ/mol, b.p.): 43.17
14. Critical temperature (ºC): 343.85
15. Solubility Parameter (J·cm-3)0.5: 18.980
16. van der Waals area (cm2·mol -1): 1.144×1010
17. Explosion upper limit (%, V/V): 1.5
18 . Lower explosion limit (%, V/V): 17.4
19. Solubility: miscible with water, alcohol, hydrocarbons, etc. It forms an azeotropic mixture with water, with an azeotropic point of 99.8°C and containing about 25% diglyme.
20. van der Waals volume (cm3·mol-1): 79.360
21. Standard heat of liquid phase Melt (J·mol-1·K-1): 272.9
Toxicological data
1. Acute toxicity: Rat oral LD50: 5400mg/kg; mouse oral LC5O: 6000mg/kg
Ecological data
Slightly harmful to water.
Molecular structure data
1. Molar refractive index: 35.08
2. Molar volume (cm3/mol): 146.6
3. Isotonic specific volume (90.2K ): 329.8
4. Surface tension (dyne/cm): 25.5
5. Polarizability (10-24cm3): 13.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 27.7
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 41.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container tightly sealed.
2. They should be stored separately from oxidants and acids, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment.
3. The storage area should be equipped with leakage emergency treatment equipment and suitable containment materials.
Synthesis method
1. Obtained from the reaction of diethanol and methanol. It can also be obtained by the reaction of diethylene glycol monomethyl ether and methyl chloride (or dimethyl sulfate).
Refining method: first dry with granular sodium hydroxide, then reflux and distill under reduced pressure in the presence of calcium hydride, lithium aluminum hydride, sodium boron hydride or sodium hydride. Operation should be carried out in nitrogen flow. If diglyme has an amine-like odor, it can be removed by shaking it with a weakly acidic ion exchange resin before drying and distillation. Add 0.01% sodium borohydride during storage to prevent the formation of peroxide.
Purpose
1. Mainly used as solvent. Used as a solvent for alkali metal hydroxides in the synthesis of metal organic compounds, alkylation reactions, polycondensation reactions and reduction reactions. Diglyme is an aprotic polar solvent that can be used as a solvent for polar organic reactions, anionic polymerization, and coordination ion polymerization.

 微信扫一扫打赏
微信扫一扫打赏

