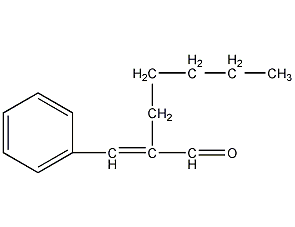
structural formula
| business number | 03ez |
|---|---|
| molecular formula | c14h18o |
| molecular weight | 202.29 |
| label |
methyl amyl cinnamaldehyde, 2-(phenylmethylene)heptanal, α-amylcinnamaldehyde, jasmonaldehyde, frantic aldehyde, fema 2061, jasminal, a-pentylcinnamaldehyde, aca, a-amyl cinnamic aldehyde, alpha-pentylcinnamaldehyde, alpha-n-amylcinnamaldehyde, alpha-n-amyl cinnamic aldehyde, food additives, food spices, flavor enhancer |
numbering system
cas number:122-40-7
mdl number:mfcd00006988
einecs number:204-541-5
rtecs number:gd6825000
brn number:none
pubchem number:24857376
physical property data
1. properties: light yellow to yellow transparent liquid 2. boiling point (℃, 2.67pa): 174~175 3. boiling point (℃, 666pa): 140 4. relative density (20℃): 0.9711
toxicological data
1. acute toxicity: rat oral ld50: 3.7g/kg; rabbit skin test ld50: >2g/kg
ecological data
none yet
molecular structure data
1. molar refractive index: 65.33
2. molar volume (cm3/mol): 210.0
3. isotonic specific volume (90.2k ): 514.2
4. surface tension (dyne/cm): 35.9
5. dielectric constant:
6. dipole moment (10-24cm3):
7. polarizability: 25.90
compute chemical data
1. hydrophobic parameter calculation reference value (xlogp): 4.2
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 1
4. number of rotatable chemical bonds: 6
5. number of tautomers:
6. topological molecular polar surface area (tpsa): 17.1
7. number of heavy atoms: 15
8. surface charge: 0
9. complexity: 199
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters quantity: 1
14. uncertain number of stereocenters of chemical bonds���: 0
15. number of covalent bond units: 1
properties and stability
none yet
storage method
packaged in brown glass bottles. store in a cool place.
synthesis method
1. it is one of the main components of jasmine oil. industrially, it is mainly produced by the condensation of heptanal and benzaldehyde obtained from castor oil. the reaction formula is as follows:
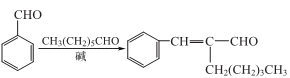
2. in add benzaldehyde to the alkali aqueous solution of methanol, and gradually add heptaldehyde at 32°c to react. then add water to the reactant, and separate the liquids to collect the oil phase (crude product). the crude product is washed with saturated brine and then distilled under reduced pressure to collect the 140-145°c (067kpa) fraction to obtain jasmonaldehyde.
3.it is obtained by the condensation of benzaldehyde and heptanaldehyde in alkaline ethanol solution.
purpose
1. used in food flavoring agents, cosmetic flavors and soap flavors.
2.used to prepare apples, strawberries, cherries, peaches, almonds, etc. essence. generally in chewing gum 15mg/kg; in baked goods 4.5mg/kg ;4.0mg/kg in candies; 1.5mg/kg in cold drinks; soft drinks medium 1.3mg/kg.
3. used in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

