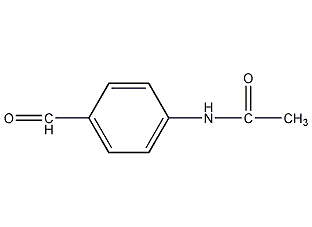
Structural formula
| Business number | 03FN |
|---|---|
| Molecular formula | C9H9NO2 |
| Molecular weight | 163.17 |
| label |
Acetaminobenzaldehyde, 4-acetamidobenzaldehyde, 4′-Formylacetanilide, CH3CONHC6H4CHO, aromatic compounds |
Numbering system
CAS number:122-85-0
MDL number:MFCD00003380
EINECS number:204-579-2
RTECS number:None
BRN number:None
PubChem number:24890589
Physical property data
1. Characteristics: yellow crystal.
2. Density (g/mL,25/4℃): Undetermined
3. Relative vapor density (g/mL,AIR=1): Undetermined
4. Melting point (ºC):156
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flash Point (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC): Undetermined
12. Saturated vapor pressure ( Isotonic specific volume (90.2K): 356.0
4. Surface tension (dyne/cm): 49.5
5. Polarizability (10-24cm3):18.74
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 9
6. Topological molecule polar surface area 46.2
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 171
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
It is obtained by acetylation of p-aminobenzaldehyde and acetic anhydride. Add acetic anhydride to the benzene solution of p-aminobenzaldehyde after azeotropic dehydration, continue distillation to recover benzene, and steam out the remaining benzene by passing water vapor. Then add water to the reaction product, and dissolve it with sodium carbonate. FONT-SIZE: 9pt; FONT-FAMILY: Verdana; font-size: 7.5pt”>pHAdjust the value to6.8-7, add activated carbon to95-100℃Decolorization, filter pressing. The filtrate is cooled and crystallized, filtered, and then filtered and dried. FONT-SIZE: 9pt; FONT-FAMILY: Verdana; mso-bidi-font-family: Tahoma; mso-bidi-font- size: 7.5pt”>60-65℃Dry to obtain paracetamidobenzaldehyde.
Purpose
Organic synthesis , used in pharmaceutical production and also as a raw material for other organic synthesis.
t-size: 7.5pt”>6.8-7, add activated carbon to95-100℃Decolorization, press filtration. The filtrate is cooled and crystallized, filtered, and 60-65 ℃Dry to obtain paracetamidobenzaldehyde.
Purpose
Organic synthesis , used in pharmaceutical production and also as a raw material for other organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

