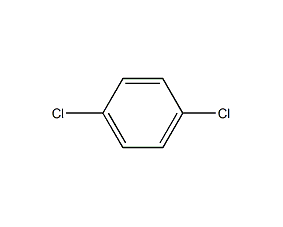
Structural formula
| Business number | 02TJ |
|---|---|
| Molecular formula | C6H4Cl2 |
| Molecular weight | 147.00 |
| label |
1,4-Dichlorobenzene, p-dichlorobenzene, p-dichlorobenzene, 1,4-Dichloorbenzene, p-Dichlorobenzene, pesticides, disinfectant, fabric moth repellent, antifungal agent, air deodorizer, Halogenated hydrocarbon solvents |
Numbering system
CAS number:106-46-7
MDL number:MFCD00000604
EINECS number:203-400-5
RTECS number:CZ4550000
BRN number:1680023
PubChem ID:None
Physical property data
1. Characteristics: white crystal with camphor smell. [1]
2. Melting point (℃): 53.1[2]
3. Boiling point (℃): 174 [3]
4. Relative density (water = 1): 1.46[4]
5. Relative vapor density (Air=1): 5.08[5]
6. Saturated vapor pressure (kPa): 1.33 (54.8℃)[6]
7. Heat of combustion (kJ/mol): -2931.3[7]
8. Critical temperature (℃): 407.5[8]
9. Critical pressure (MPa): 4.11[9]
10. Octanol/water partition coefficient: 3.37[ 10]
11. Flash point (℃): 66 (CC) [11]
12. Ignition temperature (℃) :646[12]
13. Explosion upper limit (%): 7.8[13]
14. Explosion lower limit ( %): 1.8[14]
15. Solubility: Insoluble in water, soluble in ethanol, ether, and benzene. [15]
16. Viscosity (mPa·s, 55.4ºC): 0.8394
17. Flash point (ºC, closed): 65.6
18. Flash point (ºC, open): 73.9
19. Heat of evaporation (KJ/mol): 38.81
20. Heat of fusion (KJ/mol) : 18.17
21. Heat of formation (KJ/mol, 25ºC, solid): 42.37
22. Heat of combustion (KJ/mol, 25ºC, liquid): 2936.08
23. Solubility (%, water, 35ºC): 0.010
24. Specific heat capacity (KJ/(kg·K), -50ºC, solid): 0.92
25. Specific heat capacity (KJ/(kg·K), 53~59ºC, liquid): 1.25
26. Sublimation heat α form (KJ/kg): 64.81
27. Sublimation heat β Shape (KJ/kg): 63.05
28. Refractive index at room temperature (n25): 1.521080
29. Refractive index at room temperature (n20): 1.528555
30. Relative density (25℃, 4℃): 1.241760
31. Relative density (20℃, 4℃): 1.503529
32. Liquid phase standard claims heat (enthalpy) (kJ· mol-1): -20.7
33. Liquid phase standard hot melt (J·mol-1·K-1): 170.9
34. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): 22.5
35. Gas phase standard entropy ( J·mol-1·K-1): 336.85
36.��� phase standard free energy of formation (kJ·mol-1): 76.7
37. Gas phase standard hot melt (J·mol-1· K-1):113.77
Toxicological data
1. Acute toxicity[16]
LD50: 500mg/kg (rat oral); >2g/kg (rabbit oral Skin)
LC50: 5000mg/m3 (rat inhalation, 4h)
2. Irritation[17] Human eye: 80ppm, causing irritation.
3. Subacute and chronic toxicity[18] Rats, guinea pigs and rabbits exposed to 5.23g/m3 , 69 times, tremors, weakness, weight loss, eye irritation, disheveled hair, and pathological changes in liver and kidney were seen.
4. Mutagenicity [19] Sister chromatid exchange: human lymphocytes 100μg/L. Sperm morphology: rat abdominal cavity 800mg/kg
5. Teratogenicity[20] Orally dyed female rats 6 to 15 days after conception The poison 7500mg/kg caused developmental malformations of the musculoskeletal system in offspring rats, and inhalation of 800ppm/6h in female rabbits 6 to 18 days after conception caused developmental malformations of the cardiovascular system.
6. Carcinogenicity [21] IARC Carcinogenicity Comment: G2B, suspected carcinogen in humans.
7. Others[22] The lowest oral toxic dose in rats (TDLo): 7500mg/kg (administered on 6th to 15th day of pregnancy), causing Musculoskeletal developmental abnormalities.
Ecological data
1. Ecotoxicity[23]
LC50: 4.54mg/L (24h) (bluegill sunfish, static);
7.5~10mg/L (24h), 7.17mg/L (48h), 7.4mg/L (96h) (red perch, static);
33.7mg/L (96h) ) (fathead minnow);
129mg/L (48h), 69mg/L (96h) (grass shrimp)
2. Biodegradability [ 24]
Aerobic biodegradation (h): 672~4320
Anaerobic biodegradation (h): 2688~17280
3. Non-biodegradability[25]
Photolysis maximum light absorption wavelength range (nm): 223.5~280
Photooxidation half-life in air (h): 200.6~2006
First-level hydrolysis half-life (h): >879a
4. Bioaccumulation[26] BCF: 78 (mosquito fish, contact concentration 57~233μg/L, contact time 1~4d); 370~720 (rainbow trout, contact time 119d); 60 ( Bluegill sunfish, exposure time 28 days)
5. Other harmful effects[27] This substance is harmful to the environment and can cause damage to water bodies and the atmosphere. Pollution bioaccumulates in food chains important to humans, especially in aquatic organisms.
Molecular structure data
1. Molar refractive index: 36.04
2. Molar volume (cm3/mol): 113.3
3. Isotonic specific volume (90.2K ): 279.0
4. Surface tension (dyne/cm): 36.7
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 14.28
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 54.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. This product mainly damages the liver, followed by the kidneys. Long-term exposure to air containing p-dichlorobenzene can cause symptoms such as headache, nausea, vomiting, weakness, liver atrophy, cataracts, and pain in the skin, eyes, and throat. In severe cases, it may damage the liver and internal organs, and may develop into cirrhosis of the liver. Necrosis. The olfactory threshold concentration is 0.03mg/L. The maximum allowable concentration in the workplace is 450 mg/m3 (United States) and 300 mg/m3 (Japan).
2. Stability[28] Stable
3. Incompatible substances[29] Strong oxidizer, aluminum
4. Conditions to avoid contact[30] Heating
5. Polymerization hazard[31] No polymerization
6. Decomposition products[32] Hydrogen chloride
Storage method
Storage Precautions[33] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, aluminum, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Directional chlorination of benzene or recovery from chlorobenzene production.
1. Directional chlorination of benzene. Place benzene in a chlorination reactor, add 0.1%-0.6% of the weight of benzene antimony sulfide, introduce chlorine gas, and control the chlorination temperature at about 20°C. for 30-45 minutes, add the benzene sulfonic acid directional catalyst, and then pass in chlorine gas. When the dichlorobenzene crystals precipitate, heat the reaction solution to 50-60°C, and then slowly add chlorine gas until the reaction solution increases in weight by the theoretical amount. Until about 95%, the yield is 70%-75%. Since the benzene chlorination product has many components, the key to industrial preparation is to select appropriate separation and purification methods for dichlorobenzene. There are many methods for separating and refining p- and ortho-dichlorobenzene. The distillation method is more difficult and energy-consuming than the crystallization method, which is not conducive to product stability and equipment corrosion prevention. The recrystallization method has been developed into the continuous fractional crystallization method. The emulsification method uses surface-active substances to emulsify and separate. This method has the advantages of simple operation, low energy consumption, high efficiency and less environmental pollution. Research on it is increasing and developing rapidly.
![]()
2. Production process of chlorobenzene The chlorobenzene distillation tower bottom is recovered, and the mixed dichlorobenzene is steamed out through vacuum distillation, and is crystallized in a crystallizer to obtain relatively pure p-dichlorobenzene.
Purpose
1. Used in the synthesis of bright red-based GG, reactive bright yellow and other dyes, used as pesticide intermediates, fumigation insecticides, fabric mothproofing agents, and also used in organic synthesis.
2. Used as pesticides, fungicides, analytical reagents and in organic synthesis. [34]
��Pour chlorine gas slowly until the weight of the reaction solution increases by about 95% of the theoretical amount, and the yield is 70%-75%. Since the benzene chlorination product has many components, the key to industrial preparation is to select appropriate separation and purification methods for dichlorobenzene. There are many methods for separating and refining p- and ortho-dichlorobenzene. The distillation method is more difficult and energy-consuming than the crystallization method, which is not conducive to product stability and equipment corrosion prevention. The recrystallization method has been developed into the continuous fractional crystallization method. The emulsification method uses surface-active substances to emulsify and separate. This method has the advantages of simple operation, low energy consumption, high efficiency and less environmental pollution. Research on it is increasing and developing rapidly.
![]()
2. Production process of chlorobenzene The chlorobenzene distillation tower bottom is recovered, and the mixed dichlorobenzene is steamed out through vacuum distillation, and is crystallized in a crystallizer to obtain relatively pure p-dichlorobenzene.
Purpose
1. Used in the synthesis of bright red-based GG, reactive bright yellow and other dyes, used as pesticide intermediates, fumigation insecticides, fabric mothproofing agents, and also used in organic synthesis.
2. Used as pesticides, fungicides, analytical reagents and in organic synthesis. [34]

 微信扫一扫打赏
微信扫一扫打赏

