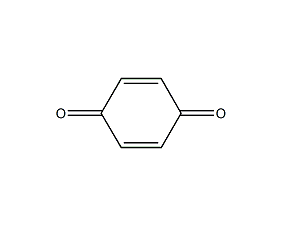
Structural formula
| Business number | 02TP |
|---|---|
| Molecular formula | C6H4O2 |
| Molecular weight | 108.09 |
| label |
Benzoquinone, benzoquinone, To quinone, 1,4-benzoquinone, 1,4-benzenedione, 1,4-Benzoquinone, 1,4-Cyclohexadiene dioxide, Quinone, 2,5-Cyclohexadiene-1,4-dione, p-Quinone, Polymerization inhibitor, dye intermediates, rubber antioxidant, polymerization initiator, chlorinating agent, Oxidizing reagent |
Numbering system
CAS number:106-51-4
MDL number:MFCD00001591
EINECS number:203-405-2
RTECS number:DK2625000
BRN number:773967
PubChem ID:None
Physical property data
1. Characteristics: Golden yellow prismatic crystals with pungent odor. [11]
2. Melting point (℃): 115.7[12]
3. Boiling point (℃): 293 (Sublimation)[13]
4. Relative density (water = 1): 1.32[14]
5. Relative vapor density (air = 1): 3.73[15]
6. Saturated vapor pressure (kPa): 0.01 (25℃)[16]
7. Critical pressure (MPa): 5.96[17]
8. Octanol/water partition coefficient: 0.2 (calculated value)[18]
9. Flash point (℃): 38~93 (CC) [19]
10. Ignition Temperature (℃): 560[20]
11. Explosion limit (%): 13.5[21]
12 .Lower explosion limit (%): 1.7[22]
13. Solubility: Slightly soluble in water, soluble in hot water, ethanol, ether, and alkali. [23]
Toxicological data
1. Acute toxicity[24] LD50: 130mg/kg (rat oral)
2. Irritation No information available
Ecological data
1. Ecotoxicity[25]
LC50: 0.125mg/L (96h) (rainbow trout); 0.045mg/L ( 96h) (fathead minnow)
EC50: 2.09mg/L (5~30min) (photobacteria, Microtox toxicity test)
2. Biodegradability [26]
Aerobic biodegradation (h): 1~120
Anaerobic biodegradation (h): 4~480
3. Non-biodegradability[27] Photooxidation half-life in air (h): 0.66~6.6
Molecular structure data
1. Molar refractive index: 27.13
2. Molar volume (cm3/mol): 8�3)[3].
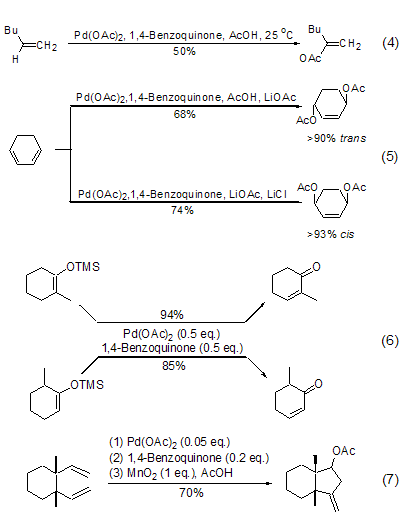
1,4-Benzoquinone The representative use is as a co-oxidant in the palladium acetate catalytic reaction to re-oxidize the Pd(0) produced after reduction and elimination into Pd(II) and enter the catalytic cycle. For example, the oxidative coupling reaction between olefins and acetic acid is achieved with 0.1 times the amount of palladium acetate and 1 times the amount of 1,4-benzoquinone (Formula 4)[4]. The 1,4-benzoquinone and palladium acetate oxidation system can also achieve the conversion of 1,3-cyclohexene into 1,4-acetoxy-2-cyclohexene with a high yield (Formula 5)[5 ], the reaction product will be affected by other adducts. For example, in the presence of lithium acetate, the main product of the reaction is trans-diacetoxy compound; in the presence of lithium acetate and lithium chloride, , the main product of the reaction is cis-diacetoxy compound.
In addition, the 1,4-benzoquinone and palladium acetate oxidation system can also realize the conversion of methylsilane enol ether to conjugated enone, and the reaction has very good regioselectivity and stereoselectivity ( Formula 6)[6]. Combining 1 part of manganese oxide reagent, 0.05 part of palladium acetate and 0.2 part of 1,4-benzoquinone can also achieve the oxidative ring-closure reaction of 1,5-hexadiene, and obtain cyclopentane derivatives with high yield (Formula 7) [7].
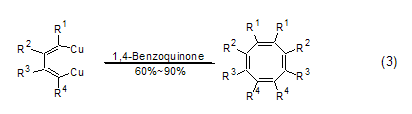
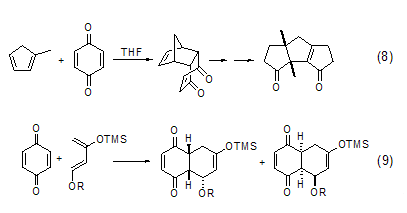
1,4-Benzoquinone Another important class of reactions is as dienophiles. Because of the electron-withdrawing effect of the carbonyl group, 1,4-benzoquinone is a good electrophile, and the Diels-Alder reaction can be easily achieved in the presence of electronegative diene substrates. A typical example is the Diels-Alder reaction of 1,4-benzoquinone and 1-methyl-1,3-cyclopentadiene in the total synthesis of Capnellene reagent, which provides a good reaction precursor for the final product ( Formula 8)[8]. In addition, there are many reports on the asymmetric Diels-Alder reaction involving 1,4-benzoquinone (Formula 9)[9].
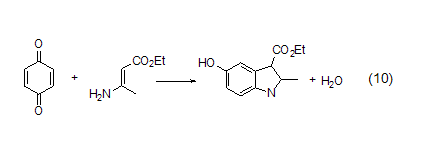
It is worth emphasizing that 1, Another important use of 4-benzoquinone is in the synthesis of 5-hydroxyindole derivatives (Formula 10)[10]. The reaction is very simple and has a wide substrate selectivity.
5. Used as dye intermediate , used in the analysis to determine amino acids. [32]

 微信扫一扫打赏
微信扫一扫打赏

