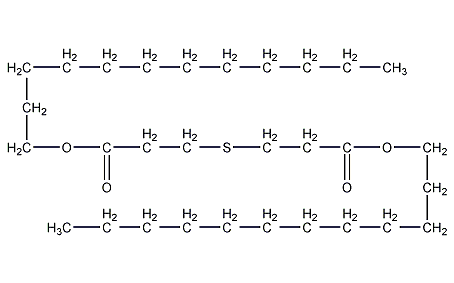
Structural formula
| Business number | 03GD |
|---|---|
| Molecular formula | C30H58O4S |
| Molecular weight | 514.84 |
| label |
Antioxidant DLTP, Dilauryl thiodipropionate, Antioxidant DLTP, Thio dipropionate didodecyl, Antioxidants |
Numbering system
CAS number:123-28-4
MDL number:MFCD00026589
EINECS number:204-614-1
RTECS number:UF8000000
BRN number:None
PubChem number:24893396
Physical property data
1. Properties: White flocculent or flaky crystalline solid.
2. Relative density (g/mL, 25/4℃): 0965
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 38~40
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): 0.2 mm Hg (163 °C)
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol) : Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Oil and water (octanol /water) distribution coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V) : Uncertain
19. Solubility: Soluble in methanol, acetone, carbon tetrachloride and benzene, insoluble in water.
Toxicological data
Acute toxicity: Rat oral LD50: >2500mg/kg; Mouse oral LC50: >2gm/kg; Mouse peritoneal membrane LC50: >2gm/kg
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 152.58
2. Molar volume (cm3/mol): 544.6
3. Isotonic specific volume (90.2K ): 1325.8
4. Surface tension (dyne/cm): 35.1
5. Dielectric constant (F/m):
6. Dipole Distance (D):
7, Polarizability (10-24cm3): 60.48
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 11.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 30
5. Number of tautomers: none
6. Topological molecule polar surface area 77.9
7. Number of heavy atoms: 35
8. Surface charge: 0
9. Complexity: 416
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. White flocculent or flaky crystalline solid. Little odor and low toxicity.
Storage method
1.200L iron drum. Prevent moisture and mechanical impurities from being mixed in. Store in a cool, ventilated and dry warehouse. Keep away from fire and heat sources.
Synthesis method
1. Acrylonitrile route
Acrylonitrile and sodium sulfide aqueous solution are condensed at 20’E: ±2°C. The reaction product is washed with water, separated, and then hydrolyzed with 55% sulfuric acid to obtain thiodipropionic acid; The precipitated and separated thiodipropionic acid is dissolved in water, and esterified with lauryl alcohol under reduced pressure under the action of sulfuric acid. The reaction product is dissolved in acetone, neutralized with sodium carbonate, filtered, crystallized, filtered, and dried to obtain the finished product.
Each ton of product consumes 700kg of acrylonitrile, 900kg of 57% sodium sulfide, 700kg of concentrated sulfuric acid, and 1100kg of lauryl alcohol.
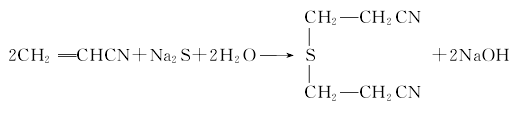
2CH2=CHCN+Na2S→S(CH=CHCOONa)2[H2SO4] →S(CH=CHCOOH)2
S(CH=CHCOOH)2+2C12H25OH→S(CH=CHCOOC12H25)2+ 2H2O
Preparation of thiodipropionitrile Add acrylonitrile to the stirred reactor, slowly add sodium sulfide aqueous solution under stirring, (Condensation reaction is carried out at 20±2)℃, and the reaction product is washed and separated with water to obtain thiodipropionitrile:
2. Acrylic route
Containing NaOH at 50℃ Add a certain amount of acrylic acid dropwise to the Na2S aqueous solution, and control the dropping speed so that the temperature is lower than 75°C. After the dropwise addition is completed, heat to boiling and reflux for 1.5 hours. After the solution cools down slightly, add 50% sulfuric acid to Ph=1. After cooling, thiodipropionic acid precipitates and is filtered to obtain a crude product with a melting point of 26°C.
Add a certain amount of lauryl alcohol to the above product, slowly heat it under vacuum until all thiodipropionic acid is dissolved, the reaction end temperature is 110°C, and the vacuum degree is greater than 0.097MPa, then add water to the mixture to make it The product precipitates; it is washed with sodium carbonate solution and water until neutral, and dried in vacuum to obtain the finished product, with a melting point of 39 to 40°C.
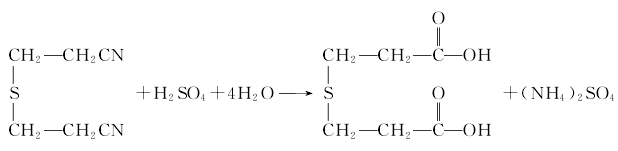
2CH2=CHCOOH+Na2S→S(CH=CHCOONa)2[H2SO4] →S(CH=CHCOOH)2
S(CH=CHCOOH)2+2C12H25OH→S(CH=CHCOOC12H25)2+ 2H2O
Preparation of thiodipropionic acid Add thiodipropionitrile to the glass-lined reaction kettle, add 55% sulfuric acid solution under stirring for hydrolysis , the reaction product is filtered to obtain thiodipropionic acid:
Preparation of antioxidant TPL Add thiodipropionic acid and water to the glass-lined reactor, stir and dissolve, add lauryl alcohol and concentrated sulfuric acid, and carry out esterification reaction under stirring to obtain crude antioxidant DLTP. The esterification reaction is operated under negative pressure and the water generated by the reaction is removed. The reaction product is dissolved in acetone, neutralized with sodium carbonate, filtered, crystallized, filtered and dried to obtain the finished product. The reaction formula is as follows: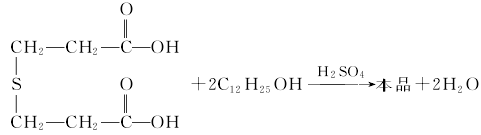
Purpose
1. This product is an excellent auxiliary antioxidant, which can decompose hydroperoxides to produce a stable structure to prevent oxidation. This product is widely used as antioxidant and stabilizer for polyethylene, polypropylene, polyvinyl chloride, ABS resin, etc. It can also be used for natural rubber and synthetic rubber. Used together with phenolic antioxidants, it can produce a synergistic effect and has excellent resistance to flex cracking. Because it is non-staining and non-polluting, it is suitable for white or brightly colored products. This product is particularly effective as a heat stabilizer in polypropylene processing. This product is also commonly used as an antioxidant in greases, soaps, lubricants and greases, and is often used in conjunction with alkylphenol antioxidants or UV absorbers. It has very low toxicity and can be used to make food packaging films.
2. The most important variety of thioester antioxidants is an excellent auxiliary antioxidant, which can decompose hydroperoxides to produce stable The structure prevents oxidation. It can protect polymers from thermo-oxidative aging and color stability under harsh conditions, and has a good synergistic effect when used in conjunction with hindered phenolic antioxidants. It is widely used as antioxidant and stabilizer for polyethylene, polystyrene, polypropylene, polyvinyl chloride, ABS resin, etc., and can also be used for natural rubber and synthetic rubber. Because it is non-staining and non-polluting, it is suitable for white or brightly colored products. Particularly effective as a heat stabilizer in polypropylene processing. It is also commonly used as an antioxidant in greases, soaps, lubricants and greases, and is often used in conjunction with alkylphenol antioxidants or UV absorbers.
3.As an auxiliary antioxidant used in food and cosmetics, the maximum reference dosage is 0.02% (based on oil content).
�Use with absorbent.
3.As an auxiliary antioxidant used in food and cosmetics, the maximum reference dosage is 0.02% (based on oil content).

 微信扫一扫打赏
微信扫一扫打赏

