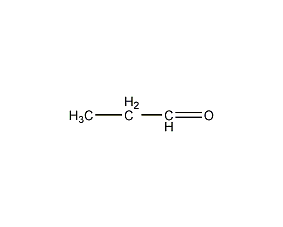
Structural formula
| Business number | 03GM |
|---|---|
| Molecular formula | C3H6O |
| Molecular weight | 58 |
| label |
n-propionaldehyde, Methyl acetaldehyde, Propanal, Methylacet aldehyde, Propyllaldehyde |
Numbering system
CAS number:123-38-6
MDL number:MFCD00007020
EINECS number:204-623-0
RTECS number:UE0350000
BRN number:506010
PubChem ID:None
Physical property data
1. Properties: Colorless and transparent liquid with pungent odor. [1]
2. Melting point (℃): -81[2]
3. Boiling point (℃): 48~49[3]
4. Relative density (water = 1): 0.80[4]
5. Relative vapor density (air=1): 2.0[5]
6. Saturated vapor pressure (kPa): 31.3 (20℃)[6]
7. Heat of combustion (kJ/mol): -1822.7[7]
8. Critical pressure (MPa): 4.65[ 8]
9. Octanol/water partition coefficient: 0.59~0.83[9]
10. Flash point (℃): -30[10]
11. Ignition temperature (℃): 207[11]
12. Explosion upper limit (%): 17.0[12]
13. Lower explosion limit (%): 2.6[13]
14. Solubility: soluble in water, miscible in most organic solvents such as ethanol and ether. [14]
15. Viscosity (mPa·s, 20ºC): 0.4
16. Solubility (%, 20ºC, water): 35
17. Specific heat capacity (KJ/(kg·K), 20ºC): 2.1856
18. Heat of vaporization (KJ/kg, 0.1MPa): 896
19 .Surface tension (mN/m, 20ºC): 21.8
20. Relative density (25℃, 4℃): 0.7912
21. Refractive index at room temperature (n25 ): 1.3593
22. Critical density (g·cm-3): 0.285
23. Critical volume (cm 3·mol-1): 204
24. Critical compression factor: 0.256
25. Eccentricity factor: 0.302
26. Solubility parameter (J·cm-3)0.5: 19.372
27. van der Waals area (cm 2·mol-1): 5.840×109
28. van der Waals volume (cm3 sup>·mol-1): 39.040
29. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -1852.4
30. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -185.6
31. Gas phase standard entropy (J·mol-1·K-1): 304.51
32. Gas phase standard free energy of formation (kJ·mol-1): -123.8
33. Vapor phase standard hot melt (J·mol-1·K-1): 80.73
34 .Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -1822.7
35. Liquid phase standard claimed heat (enthalpy) (kJ·mol -1): -215.3
36. Liquid phase standard entropy (J·mol-1·K-1): 212.30
37. Liquid phase standard free energy of formation (kJ·mol-1): -139.08
38. Liquid phase standard hot melt (J·mol -1·K-1): 129.3
Toxicological data
1. Acute poison��[15]
LD50: 1410mg/kg (rat oral); 2460mg/kg (rabbit transdermal)
LC50: 21800mg/m3 (mouse inhalation, 2h)
2. Irritation [16]
Rabbit transdermal: 500mg, mild stimulation (open stimulation test).
Rabbit eye: 41 mg, severe irritation.
3. Subacute and chronic toxicity [17] Inhalation, 90ppm, 6 hours a day for 20 days, without any obvious pathological changes; the concentration is 1300ppm, for 6 consecutive days, liver damage may occur.
Ecological data
1. Ecotoxicity[18]
EC50: 260mg/L (72h) (Scenedesmus); 134mg/L (24h) , 89mg/L (48h) (water flea)
LC50: >180mg/L (24h) (bluegill sunfish, static); 120mg/L (48h), 110mg/L (72h), 105mg/L (96h) (Moonfish, static)
2. Biodegradability [19]
Good Aerobic biodegradation (h): 24~168
Anaerobic biodegradation (h): 96~672
3. Non-biodegradability[20] Photooxidation half-life in air (h): 3.3~33
Molecular structure data
1. Molar refractive index: 16.13
2. Molar volume (cm3/mol): 75.3
3. Isotonic specific volume (90.2K ): 160.3
4. Surface tension (dyne/cm): 20.5
5. Polarizability (10-24cm3): 6.39
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 17.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: Like other aldehydes, it can react with other compounds under mild conditions without even the need for heating or catalysts.
2. Stability[21] Stable
3. Incompatible substances[22] Strong oxidizing agent, strong alkali, strong reducing agent, oxygen
4. Conditions to avoid contact[23] Heating , contact with air
5. Polymerization hazard[24] Polymerization
Storage method
Storage Precautions[25] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, reducing agents, alkalis, etc. and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Although there are many preparation methods for propionaldehyde, industrial production did not begin until the early 1950s. In 1975, Union Carbide Company of the United States built the first process for producing propionaldehyde through low-pressure oxo synthesis of ethylene using rhodium-phosphine as a catalyst. After establishing a large-scale device with an annual output of 45,000 tons, low-pressure oxo synthesis has gradually become the main method and development direction for propionaldehyde production. In 1981, of the total production capacity of propionaldehyde in the United States, Japan and Western Europe of 230,000 tons, more than 95% was produced using the low-pressure oxo synthesis method.
1. Oxo synthesis method is formed by the one-step reaction of ethylene, carbon monoxide and hydrogen. Initially, cobalt carbonyl was used as a catalyst and carried out at high pressure (14.7-19.6MPa). In recent years, a synthesis method using rhodium phosphine complex as a catalyst has been developed. The reaction temperature is 100°C and the pressure is 1.27-1.47MPa. This method produces no isomers and is easy to separate.
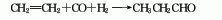
2. Propylene oxide isomerism Chemical method: 1,2-propylene oxide is obtained by gas phase isomerization in the presence of chromium vanadium catalyst. In addition, there are propanol oxidation method and acrolein hydrogenation method. When propylene is directly oxidized to produce acetone using palladium chloride as a catalyst, 0.5-1.5% of the by-product propionaldehyde is produced. When the reaction temperature of the palladium chloride content in the catalyst is increased, the proportion of propionaldehyde by-product can increase to 50%.
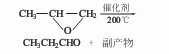
3. Preparation method:
p>
![]()
In the case of a fractionation device (accepting bottle) Cool in an ice-water bath), add 34g (0.567mol) of propanol (2) and a few grains of zeolite into a reaction flask with a dropping funnel (the bottom extends into the bottom of the bottle), and heat to boiling. Add dropwise a solution composed of 56g (0.188mol) potassium dichromate, 300mL water and 40mL concentrated sulfuric acid, and finish the addition in about 20 minutes. Keep the reaction system boiling and the temperature at the top of the fractionation column does not exceed 75°C. After all the oxidants are added, continue the reaction for 15 minutes and collect the fractions below 80°C. Separate water from the eluate, dry with anhydrous sodium sulfate, fractionate, collect fractions at 47-50°C to obtain 12g of propionaldehyde (1), with a yield of 36%. [8]
Purpose
1. Propionaldehyde is an important raw material for fine chemicals, mainly used to produce n-propanol; propionic acid; trimethylolethane; propionaldehyde oxime and other intermediates, and further to produce alkyd resins; pesticides and herbicides and Pesticides; the drugs methicillin and ethiazine are also widely used in the production of fine chemicals in coatings; plastics; food; textiles; feed; and rubber additives. It can also be used as a chain terminator for ethylene polymerization.
2. Used in synthetic resin, rubber accelerator and antioxidant. [26]
Collect the fractions at 47-50°C to obtain 12g of propionaldehyde (1), with a yield of 36%. [8]
Purpose
1. Propionaldehyde is an important raw material for fine chemicals, mainly used to produce n-propanol; propionic acid; trimethylolethane; propionaldehyde oxime and other intermediates, and further to produce alkyd resins; pesticides and herbicides and Pesticides; the drugs methicillin and ethiazine are also widely used in the production of fine chemicals in coatings; plastics; food; textiles; feed; and rubber additives. It can also be used as a chain terminator for ethylene polymerization.
2. Used in synthetic resin, rubber accelerator and antioxidant. [26]

 微信扫一扫打赏
微信扫一扫打赏

