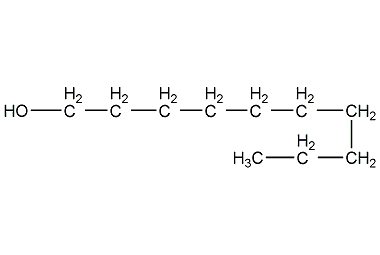
Structural formula
| Business number | 035E |
|---|---|
| Molecular formula | C10H22O |
| Molecular weight | 158 |
| label |
Decanol, decanol, Decyl oleate, 1-Decanol, 1-Decanol, N-Decanol, N-Decyl alcohol, Nonylcarbinol, Capric alcohol, capricious alcohol, Plant Growth Regulator, Aliphatic alcohols, ethers and their derivatives |
Numbering system
CAS number:112-30-1
MDL number:MFCD00004747
EINECS number:203-956-9
RTECS number:HE4375000
BRN number:1735221
PubChem number:24858475
Physical property data
1. Properties: colorless or light yellow viscous liquid with a slight smell of rose and orange blossom, and a grease and waxy smell.
2. Relative density (g/mL, 20/4℃): 0.8297
3. Relative vapor density (g/mL, air=1): 5.3
4. Freezing point (ºC): 6.9
5. Boiling point (ºC, normal pressure): 232.9
6. Refractive index (n20ºC): 1.4371
7. Flash point (ºC, open): 82
8. Vapor pressure (mmHg, 70ºC): 1
9. Saturated vapor pressure (kPa, 69.5ºC): 0.13
10. Heat of combustion (KJ/g): 41.7
11. Specific heat capacity (KJ/(kg·K), 20~30ºC, constant pressure): 2.24
12. Body expansion coefficient (K-1): 0.00086
13. Solubility: can be mixed with alcohol, ether, acetone, benzene, glacial acetic acid, cyclohexan Alkanes, carbon tetrachloride, etc. are miscible. Dissolves 0.02% in water at 20°C; water dissolves 3% in decanol.
14. Relative density (25℃, 4℃): 0.8263
15. Refractive index at room temperature (n25): 1.4353
16. Critical temperature (ºC): 405.15
17. Critical pressure (MPa): 2.315
18. Critical density (g·cm-3): 0.244
19. Critical volume (cm3·mol-1): 649
20. Critical compression Factor: 0.263
21. Eccentricity factor: 0.613
22. Solubility parameter (J·cm-3)0.5 :20.012
23. van der Waals area (cm2·mol-1): 1.573×1010
24. van der Waals volume (cm3·mol-1): 113.780
25. Gas phase standard heat of combustion ( Enthalpy) (kJ·mol-1): 6681.97
26. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -397.23
27. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -6600.47
28. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -478.73
29. Liquid phase standard hot melt (J·mol-1·K-1): 419.1
Toxicological data
1. Acute toxicity: Mouse oral LC50: 6400~12800 mg/kg; rat oral LD50: 12800~25600 mg/kg. Mouse inhalation LC50: 4000mg/m3, 2 hours.
2. Irritation number According to: skin – rabbit 20 mg/24 hours moderate; eyes – rabbit 83 mg severe.
3. It is slightly toxic and has irritating effects on eye mucosa and skin.
Ecological data
Slightly harmful to water bodies.
Molecular structure data
1. Molar refractive index: 49.91
2. Molar volume (cm3/mol): 191.1
3. Isotonic specific volume (90.2K ): 446.7
4. Surface tension (dyne/cm): 29.8
5. Polarizability (10-24cm3): 19.78
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 61.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxides. It is non-corrosive to metals and has the chemical reactivity of higher primary alcohols.
2. Almost non-toxic. The oral LD of rats is 506400~12800mg/kg, and that of mice is 12800~25600mg/kg. Mice were intraperitoneally injected with LD508800~1600mg/kg.
3. Exists in flue-cured tobacco leaves.
4. Naturally found in sweet orange oil and other essential oils.
Storage method
Stored sealed in a cool, dry place. Make sure the workspace has good ventilation. Keep away from sources of fire. Store away from oxidizing agents. Can be stored in iron, mild steel, copper or aluminum containers.
Generally, iron drums are used, and large quantities can be transported by tank trucks. Refined products can be packaged in glass bottles or iron cans. The storage tank should be grounded and use a flame arrester to prevent fire. It is advisable to protect yourself from the sun and stay away from fire and heat sources. Store and transport according to general chemical regulations.
Synthesis method
1. Natural products are made from coconut oil as raw material and are obtained by hydrogenation under high temperature and high pressure in the presence of mixed oxides. The even-numbered carbon-atom mixed alcohols (including low-carbon alcohols to octadecyl alcohols) obtained by the reaction are fractionated under reduced pressure. The C8-C12 fraction is refined by boric acid esterification. After hydrolysis, it is fractionated under reduced pressure. It can also be carbonylated from nonane. Nonaldehyde is made, then reduced to nonanol, and distilled and refined.
![]()
2. Propylene in phosphoric acid or fluorine Nonene is polymerized in the presence of boron to obtain nonene, which is obtained by reacting with carbon monoxide and hydrogen in the liquid phase.
Purpose
1. Prepare soap and daily cosmetics essence. It occupies a very important position among C8-C18 alcohols and is used in rose floral essences. As a citrus fruit flavor, it is used in beverages, candies, etc. The dosage (ppm) in food is as follows: ice cream 4.6, candy 5.2, chewing gum 3.0, and beverage 2.1. May not be used for purposes other than fragrance. Decanol is also a raw material for polyvinyl chloride wire covering materials and plasticizers (DIDP, DIDA) for high-grade artificial leather, uranium refining, defoaming agents, surfactants, and solvents. In agriculture, it can be used as a solvent and stabilizer for herbicides, pesticides and synthetic raw materials. It is used as a ripening agent for green fruits and can also be used to control the germination of ornamental plants and tobacco seeds. It can also be used in oil drilling and secondary oil recovery.

 微信扫一扫打赏
微信扫一扫打赏

