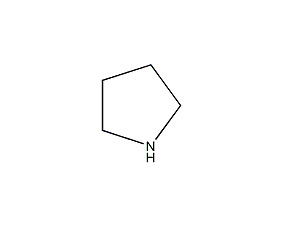
Structural formula
| Business number | 03H2 |
|---|---|
| Molecular formula | C4H9N |
| Molecular weight | 71.12 |
| label |
Methylhydrazaline, pyrrolidine, Tetrahydropyrrole, pyrrolidine, tetramethyleneimine, Pyrrolidine, Tetrahydropyrrole, Tetrahydropyrrole, Tetramethyleneimine, heterocyclic compounds, Fungicide |
Numbering system
CAS number:123-75-1
MDL number:MFCD00005249
EINECS number:204-648-7
RTECS number:UX9650000
BRN number:102395
PubChem ID:None
Physical property data
1. Properties: colorless to yellow liquid with pungent odor.
2. Boiling point (ºC, 101.3KPa): 89
3. Melting point (ºC): -63
4. Relative density (g/mL, 20/4ºC): 0.85
5. Relative vapor density (g/mL, air=1): 2.45
6. Flash point (ºC): 3
7. Vapor pressure (kPa, 39ºC): 1.8
8. Lower explosion limit (%, V/V): 2.9
9. Upper explosion limit (%, V/V ): 13.0
10. Solubility: Miscible with alcohol, ether and other organic solvents.
Toxicological data
1. Acute toxicity: Rat LD50: 300mg/Kg; Mouse LD50: 450mg/Kg
Mouse inhaled LC5O: 1300g/m 3 /2H; mice passing the peritoneal cavity LD50: 420mg/kg
The mice injected LD50: 56mg/kg through the venous vein; the rabbit mouth LDLO: 250mg/kg
The dolphin pork Jingkou mouth mouth mouth LDLO: 250mg/kg
2. It is irritating to the eyes, respiratory tract, and skin. Inhalation and absorption into the body can cause headaches, coughs, and vomiting, and may have effects on the nervous system.
Ecological data
Water hazard class 1 (German Regulation) (self-assessment via list) is slightly hazardous to water
Do not allow undiluted or large�� products come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Discharging a large amount into rivers and sewers can cause an increase in PH value. Excessively high PH value is harmful to organic matter in the water.
Deep dilution during use can greatly reduce the PH value, thereby reducing the harm to water caused by product discharge.
Molecular structure data
1. Molar refractive index: 21.79
2. Molar volume (cm3/mol): 84.7
3. Isotonic specific volume (90.2K ): 192.9
4. Surface tension (dyne/cm): 26.8
5. Polarizability (10-24cm3): 8.64
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 12
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 22.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Colorless and transparent liquid with special smell. It easily turns yellow when exposed to light or humid air. It is easily soluble in water and ethanol. Corrosive and flammable. Highly flammable, vapors form explosive mixtures with air. Emit irritating or toxic fumes when burning.
The chemical properties are alkaline, react with acids to form salts, and react violently with oxidants.
2.LD50328 mg/kg (orally administered to rats). Harmful if inhaled, ingested or absorbed through skin. Its vapor and aerosol are irritating to eyes, mucous membranes, respiratory tract and skin.
Storage method
Keep sealed and protected from light.
Closed operation, local exhaust, wear goggles, gloves, and protective clothing when working.
200kg, packed in galvanized iron drum. The warehouse should be kept cool and ventilated, away from fire, fire sources, sun protection, acids and oxidants.
Synthesis method
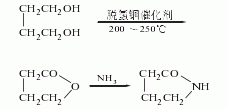
1. The butanediol method is dehydrogenated to produce γ-butyrolactone; then ammoniated to obtain it, the reaction formula is as follows:
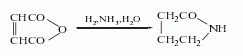
2. The maleic anhydride method uses maleic anhydride as raw material and obtains it through one-step hydrogenation and amination. The reaction formula is as follows:
Purpose
Used as pharmaceutical raw materials, organic synthesis, and special organic solvents.

 微信扫一扫打赏
微信扫一扫打赏

