Background and overview[1][2]
In the 1920s, people began to study the application of polyvinylbenzimidazole in the fields of rubber, fiber and coatings. In the late 1950s, research began on polybenzimidazole polymers containing benzimidazole groups on the polymer backbone. This type of polymer is mainly produced by the polycondensation reaction of tetraaminoaromatic compounds and dicarboxylic acid compounds. . In the 1970s, Binker and Robinson et al. conducted extensive research on the condensation of 3,3′,4,4′-biphenyltetramine and various bis-(o-diaminophenyl)alkanes with a series of aliphatic dicarboxylic acids. Research. Zhang Jian and Hou Xiaohuai of the Institute of Chemistry, Chinese Academy of Sciences used solution condensation polymerization to study the condensation of 3,3′,4,4′-tetraaminodiphenyl ether and fatty dibasic acids, and obtained polybenzoyl ether with excellent gas separation performance. imidazole membrane.
Physical and chemical properties and structure[1]
Polybenzimidazole (PBI) has received widespread attention due to its excellent mechanical properties and dielectric constant under high temperature conditions. At the same time, due to its non-combustibility and anti-fogging properties, polybenzimidazole resin is widely used in the manufacture of fibers and fabrics. Recently, some studies have introduced chelating groups into polybenzimidazole to produce ion-selective resins. Reverse osmosis membranes and hollow fibers made of polybenzimidazole have been successfully used in seawater desalination and gas separation technology. The main chain of aromatic polybenzimidazole resin contains imidazole heterocyclic groups with nitrogen atoms, thus showing excellent thermal stability. The high rigidity of the molecular chain and intramolecular interactions give polybenzimidazole a high modulus and are therefore widely used in fibers and circuit boards. In the aerospace industry, polybenzimidazole resin can be used as a structural material for spacecraft due to its good thermoplasticity and heat resistance.
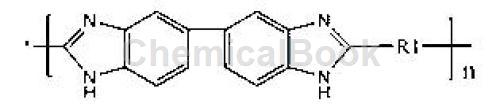
Apply[3]
Polybenzimidazole is a polymer with benzimidazole as the main repeating unit. Due to the presence of conjugated aromatic heterocycles in the molecular chain, the aromatic hexahedral arrangement of the polymer is maintained, so it has excellent thermal stability. , chemical stability and mechanical properties. Polybenzimidazole has good application prospects in the field of separation due to its excellent thermal stability and mechanical stability. In the petrochemical industry, the filter cloth or fabric made of polybenzimidazole fiber can be used for industrial product filtration, wastewater and sludge filtration, silt capture, and flue gas. And air filtration, transmission of high temperature or corrosive materials, etc. Reverse osmosis membranes and hollow fibers made of polybenzimidazole can also be used for seawater desalination and gas separation, but in the field of solvent-resistant nanofiltration, there have been no reports yet. The present invention utilizes the good thermal, mechanical and chemical stability of polybenzimidazole, improves the solvent resistance of the polybenzimidazole membrane through cross-linking, and prepares a solvent-resistant nanofiltration membrane with excellent performance.
Preparation[2]
Polybenzimidazole resin is produced by condensation polymerization of tetrabasic amines, dibasic acids and their derivatives. There are three main polymerization processes for synthesizing polybenzimidazole resins. (1) Solution polymerization; (2) Melt polymerization; (3) Nucleophilic substitution solution polymerization.
(1) Solution polymerization process
Generally, tetrabasic amine or tetraamine hydrochloride compound is added to an aprotic solvent, heated and stirred under nitrogen protection to dissolve it, then dibasic acid or its derivatives are added, heated and stirred under high temperature conditions Lower reaction. The end point of the reaction is judged based on the change in viscosity of the reactants. After the reaction is completed, the reaction mixture is poured into excess water to precipitate the prepolymer, washed and dried, and treated with high temperature for cyclization to obtain the target product. In the solution polymerization process, the solvents used are polyphosphoric acid (PPA), N,N-dimethylacetamide (DMAC), dimethyl sulfoxide (DMSO), N-methylpyrrolidone (NMP), m-cresol , N,N-dimethylpropenyl urea (DMPU), diphenyl sulfone (DPSO), etc. The most commonly used solvent is polyphosphoric acid. When using polyphosphoric acid as a solvent, there is no need to use toluene as an azeotropic dehydrating agent, because polyphosphoric acid can automatically absorb small molecules of water produced during the shrinkage and synthesis process. When using other solvents, azeotropic dehydration with toluene is required.
(2) Melt polycondensation process
Generally, the tetraamine, dibasic acid derivatives and catalyst are put into the reactor together, and under the protection of nitrogen, they are quickly heated to about 225°C while stirring, then the stirring is stopped, and the reaction temperature rises to about 270°C. And keep it for 1.5h. Cool the obtained foamed product to room temperature, grind it, then put the ground prepolymer back into the reactor, and react at about 340°C for 1 hour under the protection of nitrogen to obtain a high molecular weight polybenzimidazole resin. .
(3) Nucleophilic substitution solution condensation
The reaction process is similar to the solution condensation method, except that the reaction monomers used are different. Nucleophilic substitution solution condensation uses a tetraamino compound and a monoacid aromatic compound containing a reactive functional group such as p-hydroxybenzoic acid and p-fluorobenzoic acid to react in a solvent to synthesize a compound with a unit structure of benzimidazole, and then This compound undergoes nucleophilic substitution reaction with compounds containing F, Cl or NO2 groups to synthesize high molecular weight polybenzimidazole resin. This synthesis method can introduce ether bonds (-O-), ketone groups (-CO-), sulfone groups (-SO2-) and other groups into the main chain of polybenzimidazole polymers, thus strengthening the polymer chain. The softness improves the solubility and processability of polymers.
Main reference materials
[1] Chen Xu, Yu Xinhai, & Xu Yongfen. (2008). Research progress of polybenzimidazole. Insulating Materials, 41(6), 30-33.
[2] Lu Weifeng, & Yu Xinhai. (2000). Synthesis and application of polybenzimidazole resin. Insulating Materials (5), 5-9.
[3] Song Liyan. (2007). Synthesis and characterization of high temperature resistant material polybenzimidazole. (Doctoral dissertation, Jiangxi Normal University).

 微信扫一扫打赏
微信扫一扫打赏

