Overview[1]
O-Toluene citrate has a powerful anticholinergic structure. Because of its large hydrophobicity, it easily enters the central nervous system. It is a central anticholinergic drug and is clinically used to combat tremor paralysis. It can also be combined with other drugs to treat Parkinson’s disease and other diseases.
Pharmacological effects[1]
(1) Antihistamine effect can compete with the histamine released from tissues for receptors on effector cells, thereby preventing allergic reactions;
(2) Suppression of central nervous activity causes sedation and hypnosis;
(3) Strengthen the effect of antitussives;
(4) It also has anti-vertigo and anti-tremor paralysis effects.
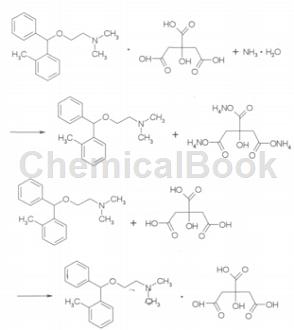
Preparation[2]
The preparation of o-toluene has been reported in patents. According to the example provided in US2567351, the experiment can be carried out by following the following process: (1) Add N, N-dimethylethanolamine to xylene , nitrogen protection, keep at a certain temperature, slowly add 2-methyldiphenylmethane chloride dropwise, and stir the reaction. After the reaction is complete, cool and there is a solid system coming out from the bottom of the reactor. Take out the supernatant and remove the solvent xylene under vacuum conditions to obtain the crude product of o-toluene; (2) Add the crude product of o-toluene to oxalic acid. Isopropyl alcohol solution generates o-toluenehydrin oxalate, filter and vacuum dry; (3) Treat o-toluenehydrin oxalate with KOH solution to obtain o-toluenehydrinate, then extract with anhydrous ether, wash with water, Dry and drain the ether to obtain pure o-toluenehydrinate. The experimental process is relatively cumbersome, the condition requirements are high, and the yield of synthesizing o-toluene is relatively low, so it is not suitable for industrial production.
Another preparation method: In a 250ml reaction bottle, add 17.5g N, N-dimethylethanolamine and 20g 2-methyldiphenylmethane respectively, stir and heat to 100°C, react for 2 hours, after the reaction is completed Cool down. The upper solution was dissolved in 140 ml of diethyl ether, washed three times with 40 ml of water, dried by adding anhydrous K2CO3, filtered, and the solvent was drained to obtain 13.6 g of the intermediate, with a yield of 56.0%. Dissolve 10.6g citric acid in 250ml isopropyl alcohol, slowly add 13.6g intermediate, stir and react for 0.5h, white crystals precipitate, wash 3 times with isopropyl alcohol, and dry to obtain 16.2g crystalline solid, with a yield of 70.0% .
Main reference materials
[1]CN200710031658.9 A kind of synthesis method of o-toluene citrate
[2] Study on the purification method of o-toluene citrate

 微信扫一扫打赏
微信扫一扫打赏

