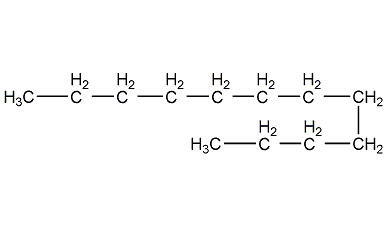
Structural formula
| Business number | 035N |
|---|---|
| Molecular formula | C12H26 |
| Molecular weight | 170.33 |
| label |
Lian Jiji, dihexyl, n-Dodecane, Bihexyl, Dihexyl, general organic chemicals, Standards and samples |
Numbering system
CAS number:112-40-3
MDL number:MFCD00008969
EINECS number:203-967-9
RTECS number:JR2125000
BRN number:1697175
PubChem ID:None
Physical property data
1. Properties: colorless liquid
2. Density (g/mL, 25℃): 0.7487
3. Solubility parameter (J·cm– 3)0.5: 15.911
4. Melting point (ºC): -9.6
5. Boiling point (ºC, normal pressure): 216.3
6. Autoignition point or ignition temperature (ºC): 200
7. Solubility: easily soluble in ethanol, ether, acetone, chloroform and carbon tetrachloride, insoluble in water.
8.van der Waals area (cm2·mol-1): 1.774×1010
9. van der Waals volume (cm3·mol-1): 129.640
10. Critical temperature (K): 384.85
11. Critical pressure (MPa): 1.82
12. Critical density (g·cm-3): 0.226
13. Critical volume (cm3·mol-1): 754
14. Critical compression factor: 0.251
15 .Eccentricity factor: 0.573
16. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -8148.26
17. Gas phase standard claim Heat (enthalpy) (kJ·mol-1): -289.66
18. Gas phase standard entropy (J·mol-1·K-1): 624.2
19. Gas phase standard free energy of formation (kJ·mol-1): 50.83
20. Gas phase Standard hot melt (J·mol-1·K-1): 278
21. Liquid phase standard hot melt (J·mol-1·K-1): 376
22. Liquid phase standard entropy (J·mol-1·K-1): 490.38
23. Liquid phase standard free energy of formation (kJ·mol-1): 29.29
24 .Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -8086.96
25. Liquid phase standard claimed heat (enthalpy) (kJ·mol -1):-350.95
Toxicological data
1. Irritation: Rabbit transdermal: 50ul/24H Moderate irritation
2. Acute toxicity: Rabbit inhalation LC50: >142ppm/8H
Ecological data
No harm to water bodies.
Molecular structure data
1. Molar refractive index: 57.64
2. Molar volume (cm3/mol): 226.6
3. Isotonic specific volume (90.2K ): 509.5
4. Surface tension (dyne/cm): 25.5
5. Dielectric constant (F/m): 2.01
6. Electrode Chemical rate (10-24cm3): 22.85
Compute chemical data
1. Hydrophobic parameter calculation reference�(XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Rotatable chemical bonds Number: 9
5. Number of tautomers: None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 56.4
10. Number of isotope atoms: 0
11. Determine the atoms Number of stereocenters: 0
12. Uncertain number of stereocenters of atoms: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxides.
Storage method
Stored sealed in a cool, dry place. Make sure the workspace has good ventilation. Keep away from sources of fire. Store away from oxidizing agents.
Synthesis method
1. Preparation method:
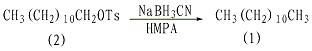
In a dry reaction bottle equipped with a stirrer, thermometer, and reflux condenser (installed with a calcium chloride drying tube), add 50 mL of hexamethylphosphoramide (HMPA), n-12 p-toluenesulfonic acid Alkyl ester (2) 6.8g (0.0201mol), sodium cyanoborohydride ① 5.02g (0.08mol). React at 80°C for 2 hours with stirring. After cooling, add 50 mL of water and extract three times with n-hexane. Combine the organic extracts, wash twice with water, dry over anhydrous magnesium sulfate, and distill under reduced pressure. Collect the 79-81°C/1.86KPa fraction to obtain 2.5-2.65g of n-dodecane (1), with a yield of 79%-81 %. Note: ① Using sodium cyanoborohydride to reduce alkyl halides and sulfonate esters in hexamethylphosphoramide is a convenient method for preparing alkanes with high selectivity. Other functional groups in the molecule such as carboxyl, carboxylate, cyanide Groups, nitro groups, amide groups, carbonyl groups, oxirane groups, etc. are not affected. [1]
2. Preparation method:
![]()
Use 23g (1.0mol) sodium metal and 82.5g (62mL, 0.5mol) 1-bromohexane (2) or 99g (65.5mL, 0.5mol) 1-Bromopentane was prepared according to the above operation method for n-octane, and the fraction at 94°C/174KPa was collected to obtain about 37g n-decane (1), with a yield of 87%. [2]
Purpose
Used as organic synthesis intermediates; solvents and standard materials for chromatographic analysis.

 微信扫一扫打赏
微信扫一扫打赏

