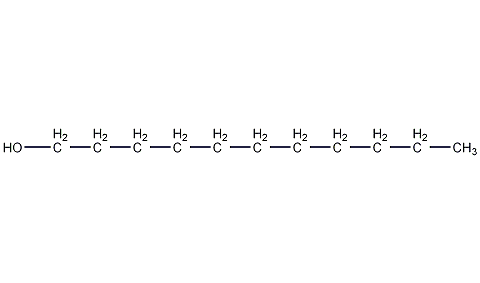
Structural formula
| Business number | 035Q |
|---|---|
| Molecular formula | C11H24O |
| Molecular weight | 172.31 |
| label |
undecyl alcohol, Decylmethanol, n-Undecanol, undecyl alcohol, undecyl alcohol, n-Undecyl alcohol, artificial flavors, alcohol solvent |
Numbering system
CAS number:112-42-5
MDL number:MFCD00004751
EINECS number:203-970-5
RTECS number:YQ315500
BRN number:1698334
PubChem number:24900621
Physical property data
1. Properties: Colorless or light yellow liquid with lemon scent.
2. Relative density (g/mL, 20/4℃): 0.8324
3. Relative density (25℃, 4℃): 0.8291
4. Freezing point (ºC): 15.9
5. Boiling point (ºC, 101.3kPa): 242.8
6. Boiling point (ºC, 2.0KPa): 131
7. Refractive index (n20ºC): 1.4402
8. Flash point (ºC, closed): 113
9. Refractive index at room temperature (n25 ): 1.4386
10. Critical temperature (ºC): 430.45
11. Critical pressure (MPa): 2.147
12. Critical density (g·cm-3): 0.240
13. Critical volume (cm3·mol-1): 718
14. Critical compression factor: 0.264
15. Eccentricity factor: 0.587
16. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -7253.72
17. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -504.88
18. Liquid phase standard hot melt (J·mol-1·K-1): 438.9
19. Solubility: insoluble Soluble in water, alcohol and ether.
Toxicological data
1. Irritation: Rabbit transdermal: 500mg/24H Moderate irritation
2. Acute toxicity: Rat oral LD5O: 3000mg/kg
Guinea pig transdermal LD50 :>20ml/kg/
Rabbit transdermal LD50: 4760ul/kg
Ecological data
It is harmful to water bodies and highly toxic to fish.
Molecular structure data
1. Molar refractive index: 54.54
2. Molar volume (cm3/mol): 207.6
3. Isotonic specific volume (90.2K ): 486.5
4. Surface tension (dyne/cm): 30.1
5. Polarizability (10-24cm3): 21.62
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 9
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 71.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxides.
Flammable liquid, non-corrosive to metals. Typical chemical reactivity of higher primary alcohols. In the presence of a catalyst, it esterifies with acid to form ester. Oxidative conversion to aldehydes or acids. Dehydration to alkenes in the presence of aluminum oxide. The vapor pressure is low and it is not dangerous to use under normal conditions.
Storage method
Stored sealed in a cool, dry place. Make sure the workspace has good ventilation. Keep away from sources of fire. Store away from oxidizing agents. Can be stored in iron, mild steel, copper or aluminum containers.
Synthesis method
It is produced by the reduction of ethyl undecanoate by metal sodium, or by the pressurized catalytic hydrogenation reduction of ω-ethyl undecenoate, or even by the Grignard reaction of n-nonylmagnesium bromide and ethylene oxide. There are also preparations using undedehyde lithium aluminum hydrogen reduction method.
Purpose
The application of this alcohol has certain limitations. Since fats and oils are solid at low temperatures, they are only used in more characteristic fragrances. Citrus rose-type alcohols only play a secondary role in coordination and are used as body fragrance in fragrances. Spices used to make acacia, moon incense and other fragrances.

 微信扫一扫打赏
微信扫一扫打赏

