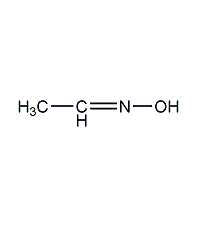
Structural formula
| Business number | 02V1 |
|---|---|
| Molecular formula | C2H5NO |
| Molecular weight | 59 |
| label |
Ethyl hydroxylamine, Acetaldehyde oxime, Aldoxime, Ethylidenehydroxylamine |
Numbering system
CAS number:107-29-9
MDL number:MFCD00002124
EINECS number:203-479-6
RTECS number:AB2975000
BRN number:1209252
PubChem number:24844978
Physical property data
1. Properties: α type is white solid, β type is white liquid.
2. Density (g/mL, 25℃): 0.97
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 46 (α type)
5. Boiling point (ºC, normal pressure): 115
6. Boiling point (ºC, kPa): Undetermined
7. Refractive index (D20): 1.4260
8. Flash point (ºC): 40
9. Specific rotation (ºC): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC ): Undetermined
12. Saturated vapor pressure (kPa, 20 ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): 52.0
18. Explosion lower limit (%, V/V): 4.2
19 . Solubility: Soluble in water, acetic acid and ether.
Toxicological data
1. Acute toxicity: mouse intraperitoneal LD50: 100mg/kg; mouse LD50: 1150mg/kg;
2. Mutagenicity
Mouse lymphocytes Gene mutation: 230mg/L;
Mouse lymphocyte mutation: 15mg/L;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 15.47
2. Molar volume (cm3/mol): 64.7
3. Isotonic specific volume (90.2K ): 149.4
4. Surface tension (dyne/cm): 28.3
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 6.13
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -0.1
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
p>
4.Number of rotatable chemical bonds: 0
5, Number of tautomers: 2
6, Topological molecular polar surface area (TPSA): 32.6
7 , Number of heavy atoms: 4
8, Surface charge: 0
9, Complexity: 25.2
10, Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 1
p>
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants and acids.
2. Flammable, irritating to eyes, respiratory system and skin. Appropriate protective clothing should be worn during heavy use. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. Store sealed and protected from light.
Synthesis method
Aldoxime is produced by the reaction of hydroxylamine and acetaldehyde aqueous solution. Reaction equation: CH3CHO+NH2OH·HCl+NaOH→CH3CH=NOH+NaCl+H2O
In current industrial production, crystalline hydroxylamine sulfate with a high content is generally used as raw material. When used, it is formulated into a 20% to 50% aqueous solution, and then reacts with acetaldehyde to generate acetaldehyde. oxime. The reaction equation is: CH3CHO+(NH2OH)2·H2SO4→CH3CH=NOH
Add acetaldehyde to the above hydroxylamine sulfate aqueous solution, react at 40-50°C for 2 hours, remove the inorganic salt after cooling, and obtain acetaldehyde containing acetaldehyde. It is a clear and transparent aqueous solution of about 50% aldoxime. After testing, except for acetaldoxime and water, the amount of impurities in this aqueous solution is very small. The yield of acetaldoxime is also more than 90% (based on hydroxylamine sulfate). This kind of aqueous solution is The aqueous solution is extracted and refined to obtain acetaldehyde oxime products with a content of more than 90%.
Purpose
1. After melting, it serves as an excellent solvent for many inorganic and organic compounds, organic synthesis, stability, plasticizer, and alcohol denaturant.
2. Aldoxime generates amide in one pot.
In the presence of the catalyst InCl3, acetaldoxime replaces water and reacts with nitrile hydration to form amides with a high yield.
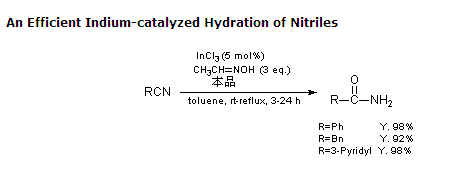

 微信扫一扫打赏
微信扫一扫打赏

