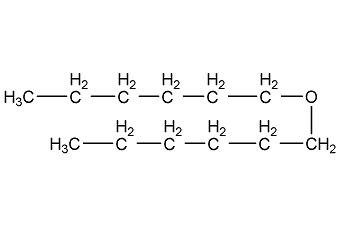
Structural formula
| Business number | 0365 |
|---|---|
| Molecular formula | C12H26O |
| Molecular weight | 186.33 |
| label |
dihexyl ether, hexyl ether, Dihexyl ether, Dihexyl ether, Bis(1-hexyl)ether, Extracting agent, Ether solvents, Defoaming agent |
Numbering system
CAS number:112-58-3
MDL number:MFCD00009525
EINECS number:203-987-8
RTECS number:KN8600000
BRN number:1738177
PubChem number:24855815
Physical property data
1. Properties: colorless liquid with slight ether smell.
2. Boiling point (ºC, 101.3kPa): 225.7
3. Boiling point (ºC, 6.67kPa): 136
4. Boiling point (ºC, 1.33 kPa): 100
5. Melting point (ºC): -43.0
6. Relative density (g/mL, 20/20ºC): 0.7942
7 . Relative density (g/mL, 20/4ºC): 0.7932
8. Relative vapor density (g/mL, air=1): 6.4
9. Refractive index (n20ºC ): 1.4207
10. Viscosity (mPa·s, 20ºC): 1.68
11. Flash point (ºC, opening): 76.7
12. Fire point (ºC): 187.2
13. Vapor pressure (kPa, 20ºC): 0.0093
14. Lower explosion limit (%, V/V): 0.6
15. Body expansion coefficient (K-1): 0.00096
16. Solubility: Slightly soluble in water, miscible with a variety of organic solvents. Dissolves 0.01% in water at 20°C; water dissolves 0.12% in hexyl ether.
17. Relative density (25℃, 4℃): 0.7894
18. Refractive index at room temperature (n25): 1.4187
Toxicological data
1. Irritation: Rabbit transdermal: open irritation test, 10mg/24 hours, severe irritation.
Rabbit transdermal: 20mg/24 hours, moderate irritation.
Rabbit eyes: open stimulation test, 500mg, caused irritation.
Rabbit eye: 500mg/24 hours, mild irritation��.
2. Acute toxicity: rat oral LD50: 30900mg/kg; rabbit dermal LD50: 5480mg/kg
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 59.38
2. Molar volume (cm3/mol): 232.9
3. Isotonic specific volume (90.2K ): 529.2
4. Surface tension (dyne/cm): 26.6
5. Polarizability (10-24cm3): 23.54
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 4.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 10
5. Number of tautomers: none
6. Topological molecule polar surface area 9.2
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 71.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It does not decompose under normal temperature and pressure. Avoid contact with strong oxidants.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Refining method: After washing with 10% sodium bisulfite, 10% sodium hydroxide, and saturated sodium chloride solution, dry with calcium chloride, metallic sodium, etc., and distill.
2. Preparation method:
![]()
Refer to the above preparation method of n-amyl ether (reference book page 242), use 68g of 1-hexanol (2) (83mL, 0.67mol, bp156~157℃), 4.5mL of concentrated sulfuric acid, and a few grains of zeolite , the temperature rises to 180°C. After the reaction is completed, cool and pour into water. Separate the organic layer, wash twice with 5% sodium hydroxide solution and once with water, dry with anhydrous potassium carbonate, and distill. Collect the fractions of 160 to 221°C (23g) and 221 to 223°C (23g). After treatment with 5g of metallic sodium and distillation, 26g of diisoamyl ether (1) was obtained with a yield of 43%. [1]
Purpose
Used as reaction medium and defoaming agent. Used as solvent and defoaming agent in chemical reactions. Used in collodion, extractants, photographic film and smokeless gunpowder, etc.

 微信扫一扫打赏
微信扫一扫打赏

