Background and overview[1]
Florfenicol, also known as fluprofen and flufenicol, is a new veterinary chloramphenicol-type broad-spectrum antibacterial drug successfully developed in the late 1980s. It was first launched in Japan in 1993. In 1993, Norway approved the drug to treat salmon boils. In 1995, France, the United Kingdom, Austria, Mexico and Spain approved it for the treatment of bovine respiratory bacterial diseases. It is also approved for use as a feed additive for pigs in Japan and Mexico to prevent and treat bacterial diseases in pigs. my country has currently approved the drug and is approved for use in bacterial infections in pigs, cattle, chickens and fish.
Forfenicol is available in powder, premix, solution and injection forms. However, the solubility of florfenicol in water is very low, which limits direct administration in drinking water. When the solution is poured into water, it will easily precipitate and block the pipeline. At present, the problem of drug insolubility in water cannot be solved through ordinary preparation methods. Currently, there is a method abroad to transform florfenicol into phosphate ester, which improves the water solubility. However, the synthesis conditions are harsh, the reaction process is dangerous and the cost is high.
The existing method for preparing florfenicol sodium succinate is: add florfenicol and the catalyst DMAP or triethylamine to acetonitrile, acetone or petroleum ether, then add succinic anhydride, and heat to 50 degrees Carry out the reaction, evaporate the organic solvent to dryness, and then react with ethanol solution of sodium hydroxide to generate sodium florfenicol succinate. This method has the following shortcomings: the catalyst DMAP and triethylamine are expensive, triethylamine has a foul smell, and the operating environment is poor. Triethylamine is difficult to separate from the organic solvent, making recovery difficult. Evaporating the organic solvent to dryness is more difficult in industrial production. It is difficult to operate, the operation of replacing other solvents is cumbersome, and the amount of organic solvents is too much. Moreover, the method has complex operation steps, expensive raw materials, excessive amounts of organic solvents and catalysts, which leads to high costs and pollution, and the solvent needs to be evaporated to dryness and replaced with other solvents. The problem of cumbersome operation in salt-forming reaction.
Apply[2]
Florfenicol is a new type of amide alcohol antibacterial drug for animals. It was successfully developed by Schering-Plough Company in the United States in the late 1980s. It has a wide antibacterial spectrum, strong bactericidal effect and remarkable curative effect, especially It does not have the potential to cause aplastic anemia due to chloramphenicol antibiotics, and is the drug of choice for the treatment of various infections caused by Pasteurella multocida, Actinobacillus pleuropneumoniae, Escherichia coli and Salmonella typhi. my country has approved the use of florfenicol for bacterial infections in pigs, cattle, chickens, fish and other animals. Its dosage forms include premix, powder, soluble powder, solution and injection. However, florfenicol is almost insoluble in water, which greatly limits its clinical application.
According to reports, there are two types of methods to improve the water solubility of florfenicol: one is physical methods, and the other is chemical methods; physical methods are mainly to make inclusion compounds or solid dispersions, but these methods have a slow dissolution rate , low dissolution, and does not fundamentally solve the problem of being difficult to dissolve in water; the chemical method is to make florfenicol into a prodrug (florfenicol sodium succinate), which is metabolized in the body to release the original drug and exert its medicinal effect. Effective, completely solving the problem of florfenicol being insoluble in water.
Sodium florfenicol succinate has good water solubility. After experimental research, the solubility of sodium florfenicol succinate in water is greater than 50g. It has weak antibacterial activity in vitro and is quickly converted into florfenicol in the body. Tests conducted in rats found that florfenicol sodium succinate has a good inhibitory effect on Staphylococcus aureus, Bacillus subtilis, Proteus, and Escherichia coli, and exerts a good antibacterial effect.
Preparation[1,3]
Method 1: Preparation method of florfenicol sodium succinate, adopt the following steps, add florfenicol, succinic anhydride and phase transfer catalyst to the organic solvent, stir, control the temperature at -10~30°C, and then Add the aqueous alkali solution dropwise to the above mixed solution, maintain stirring at -10~30°C for a period of time, and then separate the liquids to obtain an aqueous solution of florfenicol sodium succinate. Spray dry the aqueous solution to obtain florfenicol. Sodium succinate, the base is selected from one or more of sodium hydroxide, sodium carbonate, sodium bicarbonate, sodium formate, sodium acetate, sodium propionate, and sodium butyrate. Preferably, the reaction temperature is controlled at -5 to 10°C.
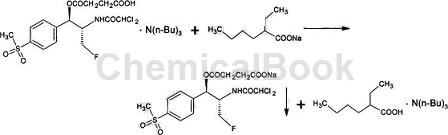
Method 2: Put 30g of florfenicol (0.084mol), 10.9g of succinic anhydride (0.109mol) and 135ml of n-hexane into a dry reaction bottle, stir at room temperature, mix thoroughly, and then cool to 5℃ , slowly add 15.5g (0.084mol) of tri-n-butylamine dropwise, and stir the reaction at 5 to 10°C. TLC spot plate tracking confirms that there is no florfenicol raw material point, indicating that the esterification reaction is completed. Then, slowly drop the ethyl acetate solution of sodium isooctanoate at 5°C, stir and react for 3 hours at 5-10°C, separate the solid and liquid, rinse the filter cake with a small amount of frozen ethyl acetate, and vacuum dry at 45°C to obtain fluorobenzene. 36.0g of sodium nicosuccinate white crystals, the content is 99.3%, and the molar yield is 89.5%.
Main reference materials
[1] CN201410046294.1 Preparation method of florfenicol sodium succinate
[2] CN201410046428.X Florfenicol sodium succinate soluble powder and its preparation method and application
[3] CN200910100938.X Preparation of florfenicol amber by one-pot method�Sodium method

 微信扫一扫打赏
微信扫一扫打赏

