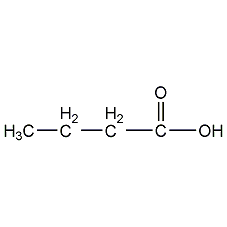
Structural formula
| Business number | 02W0 |
|---|---|
| Molecular formula | C4H8O2 |
| Molecular weight | 88.11 |
| label |
butyric acid, Killing chrysanthemic acid, n-butyric acid, propylformic acid, Ethyl acetic acid, Ethyl acetic acid, Tetranoic Acid, n-Butanoic Acid, Propyl formic acid, n-Butyric acid, emulsifier, fungicides, Extracting agent, decalcifying agent, acidic solvent |
Numbering system
CAS number:107-92-6
MDL number:MFCD00002814
EINECS number:203-532-3
RTECS number:ES5425000
BRN number:906770
PubChem number:24851531
Physical property data
1. Properties: oily liquid with rancid sour smell. [1]
2. Melting point (℃): -7.9[2]
3. Boiling point (℃): 163.5[3]
4. Relative density (water = 1): 0.96[4]
5. Relative vapor Density (air=1): 3.04[5]
6. Saturated vapor pressure (kPa): 0.10 (25℃)[6]
7. Heat of combustion (kJ/mol): -2181.4[7]
8. Critical temperature (℃): 355[8]
9. Critical pressure (MPa): 5.27[9]
10. Octanol/water partition coefficient: 0.79 [10]
11. Flash point (℃): 72 (CC) [11]
12. Ignition temperature (℃) ): 452[12]
13. Explosion upper limit (%): 10.0[13]
14. Explosion lower limit (%): 2.0[14]
15. Solubility: miscible with water, miscible with ethanol and ether. [15]
16. Refractive index (20ºC): 1.3980
17. Refractive index (25ºC): 1.3958
18 . Viscosity (mPa·s, 15ºC): 1.814
19. Viscosity (mPa·s, 30ºC): 1.385×10-3
20. Ignition point (ºC): 452.2
21. Heat of evaporation (KJ/mol, 25ºC): 60.58
22. Heat of evaporation (KJ/mol, b.p.): 42.03
23. Heat of fusion (KJ/mol): 10.47
24. Heat of formation (KJ/mol, 25ºC, liquid): -535.49
25. Specific heat capacity (KJ/ (kg·K), 20ºC, constant pressure): 1.98
26. Thermal conductivity (W/(m·K)): 0.1477
27. Critical density (g· cm-3): 0.302
28. Critical volume (cm3·mol-1): 292 p>
29. Critical compression factor: 0.232
30. Eccentricity factor: 0.604
31. Solubility parameter (J·cm-3 )0.5: 20.263
32. van der Waals area (cm2·mol-1): 7.880×10 9
33. van der Waals volume (cm3·mol-1
3. Preparation method:
In a reaction flask equipped with a stirrer, reflux condenser, thermometer and dropping funnel, add heavy 25g sodium chromate, 100mL water, 20mL concentrated sulfuric acid. Heat to 80~85℃ with stirring. Slowly add 7.5g (0.1mol) n-butanol (2) dropwise, and control the dropping speed so that white smoke does not appear in the reaction bottle. Take out the condenser and complete the addition in about 20 minutes. The reaction is exothermic and the reaction temperature is controlled to 95~100°C. After the addition, continue the reaction for 20 minutes. At the end of the reaction, there will be no white smoke in the condenser. Distill immediately after the reaction is completed and collect about 60~ 80mL distillate. Add 4g sodium hydroxide to the distillate and reflux for 0.5h. Cool to room temperature and extract twice with diethyl ether to remove unreacted butanol. Acidify the aqueous layer with sulfuric acid to strong acidity. Extract with diethyl ether (15mL× 3). Combine the ether layers and dry over anhydrous calcium chloride. Evaporate the ether in a water bath and then distill. Collect the 158-164°C fraction ① to obtain 6g of colorless n-butyric acid (1). The rate is 68%. Note: ① The previous fraction is re-distilled to obtain a small amount of 158-164°C fraction. []
Purpose
1. Used in the manufacture of butyrate esters, cellulose butyrate, varnish, drugs, spices, etc. Also used as emulsifier, bactericide and extractant. Used as a decalcifying agent in leather tanning. When measuring copper by electrolysis, it is used to eliminate the influence of iron.
2. Butyric acid is an edible spice allowed to be used in my country and is often used to add flavor to butter, cheese and fruit flavors. The dosage is based on normal production needs, generally 82 mg/kg in candies; 60 to 270 mg/kg in chewing gum; 32 mg/kg in baked goods; 18 mg/kg in margarine; and 6.5 mg/kg in cold drinks.
3. Used as extraction agent, decalcifying agent, ester synthesis, and also used to prepare spices, fungicides, emulsifiers, etc. [24]

 微信扫一扫打赏
微信扫一扫打赏

