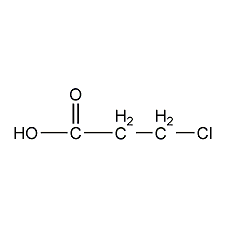
Structural formula
| Business number | 02W2 |
|---|---|
| Molecular formula | C3H5ClO2 |
| Molecular weight | 108.52 |
| label |
β-chloropropionic acid, β-Chlorooleic acid, β-Chloropropionic acid, Beta-Monochloropropionic Acid, ClCH2CH2COOH |
Numbering system
CAS number:107-94-8
MDL number:MFCD00002764
EINECS number:203-534-4
RTECS number:UE8750000
BRN number:1098495
PubChem number:24848067
Physical property data
1. Properties: White needle-like crystals, hygroscopic. [1]
2. Melting point (℃): 38~41[2]
3. Boiling point (℃) : 203~205[3]
4. Octanol/water partition coefficient: 0.41[4]
5. Flash point (℃): >110[5]
6. Solubility: soluble in water, soluble in ethanol, ether, and chloroform. [6]
Toxicological data
1. Acute toxicity[7] LD50: >2000mg/kg (oral in mice)
2. Irritation No information available
3. Mutagenicity[8] Microbial mutagenicity: Salmonella typhimurium 100μg/dish
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 22.36
2. Molar volume (cm3/mol): 84.0
3. Isotonic specific volume (90.2K ): 211.7
4. Surface tension (dyne/cm): 40.2
5. Polarizability (10-24cm3): 8.86
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 52.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[9] Stability
2. Incompatible substances [10] Strong oxidants, alkalis
3. Conditions to avoid contact[11] Heating
4. Polymerization hazard[12] No polymerization
5. Decomposition products[13] Hydrogen chloride
Storage method
Storage NotesMatters[14] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. There are many preparation methods. Propionic acid is chlorinated at about 20°C and under ultraviolet light irradiation to obtain 3-chloropropionic acid; a very small amount of hydroquinone can also be added to acrylic acid, and then dry hydrogen chloride is introduced below 20°C for reaction. The finished product is obtained by distillation under reduced pressure. In addition, acrylonitrile was added to the hot hydrochloric acid and the reaction was refluxed for 6 hours. Cool the reactant, filter out the generated ammonium chloride, extract it with diethyl ether, add 20% ammonium chloride solution to wash away the hydrochloric acid, dry it, recover the diethyl ether, and distill under reduced pressure to obtain 3-chloropropionic acid. Acrolein can also be reacted with hydrogen chloride to generate chloropropionaldehyde, which can then be oxidized with nitric acid, or 3-chloropropionic acid can be prepared by oxidizing 3-chloro-1-propanol with nitric acid.
2. Preparation method:
Add concentrated nitric acid ( d1.42) 880g (about 10mol), while cooling in an ice-water bath, add 200g (2.12mol) β-chloropropanol-1(2) dropwise, control the reaction temperature at 25~30°C, and complete the addition in about 2.5 hours. After adding, continue stirring to react 0.5g. Let sit overnight. Heat the reaction in a boiling water bath for 1 hour. Then it was changed to a vacuum distillation device, and the fractions before 100℃/2.67kPa were first evaporated (mainly dilute nitric acid, about 500mL), and then the fractions between 107~109℃/2.67kPa were collected, and solidified after cooling, mp37~39℃, About 180 g of β-chloropropionic acid (1) was obtained, with a yield of 78%. [16]
Purpose
1. Organic synthesis intermediates for the production of anti-epileptic drugs.
2. Used in organic synthesis. [15]

 微信扫一扫打赏
微信扫一扫打赏

