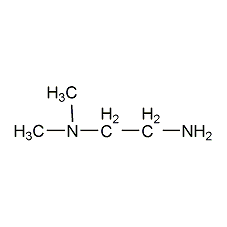
Structural formula
| Business number | 02W7 |
|---|---|
| Molecular formula | C4H12N2 |
| Molecular weight | 88.15 |
| label |
dimethylethylenediamine |
Numbering system
CAS number:108-00-9
MDL number:MFCD00008175
EINECS number:203-541-2
RTECS number:KH8608250
BRN number:605279
PubChem number:24864328
Physical property data
1. Properties: Transparent liquid
2. Relative density: 0.803
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Undetermined
5. Boiling point (ºC, normal pressure): 104~106
6. Boiling point (ºC, 12mmHg): Undetermined Confirm
7. Refractive index (D20): 1.4260~1.4300
8. Flash point (ºC): 23
9. Specific rotation (ºC): Not determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil-water (octanol/water) distribution coefficient Log value: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Undetermined
Toxicological data
1. Acute toxicity: Rat oral LD5O: 1135mg/kg
Mouse oral LD5O: 1200mg/kg
Rat inhalation LC5O: 2560mg/m3
p>
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 27.83
2. Molar volume (cm3/mol): 105.4
3. Isotonic specific volume (90.2K ): 246.0
4. Surface tension (dyne/cm): 29.5
5. Polarizability (10-24cm3): 11.03
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.7
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
p>
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 29.3
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 26.7
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides and acids.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire, heat and water sources. They should be stored separately from oxidants and acidic substances, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Feeding ratio (mass) 2-chloroethylamine hydrochloride: 40% dimethylamine: caustic soda: filter aid: caustic soda = 1: 3.88: 1.85: 0.22: 0.08.
Add 10% dimethylamine aqueous solution into the glass-lined reactor, cool to 15°C with stirring, and add 2-chloroethylamine hydrochloride in batches within 0.5 to 1 hour, with the temperature not exceeding 20°C. . After the addition is completed, let the reaction solution naturally heat up to 37°C. Then slowly cool it to 30°C, stop cooling, and then let the material liquid continue to naturally heat up to 38°C. Then cool the material liquid to 30°C, and then heat it up to 62°C. The hydrogen chloride released from the tail gas is absorbed by water or alkali. After the reaction mixture was stirred at 62~65°C for 3 hours, it was cooled to 20°C, and caustic soda was added in batches within 0.5~1h. The temperature of the material liquid was controlled at (25±5)°C. After adding the caustic soda, continue stirring at (25±5)°C for 1 hour, then let it stand for layering. Then the organic layer is separated, and the water layer is an emulsion. Diatomaceous earth needs to be added for filtration. The resulting filtrate is clarified and then separated into layers. Then combine the organic layers, add caustic soda, dry, and filter. The filtrate is distilled, unreacted dimethylamine is first recovered, and finally the fraction at 104~106°C is collected, that is, N,N-dimethylethylenediamine. The content is greater than 97%, and the yield is greater than 75%.
Purpose
This product is mainly used for the synthesis of 1-dimethylaminoethyl-5-mercaptotetrazole, the intermediate of Cefotiam.

 微信扫一扫打赏
微信扫一扫打赏

