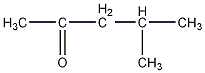
Structural formula
| Business number | 02WD |
|---|---|
| Molecular formula | C6H12O |
| Molecular weight | 100.16 |
| label |
Methyl isobutyl ketone, 2-methyl-4-pentanone, 2-isohexanone, Isopropylacetone, Isobutyl methyl ketone |
Numbering system
CAS number:108-10-1
MDL number:MFCD00008938
EINECS number:203-550-1
RTECS number:SA9275000
BRN number:605399
PubChem number:24857648
Physical property data
1. Properties: colorless and transparent liquid with a pleasant ketone-like aroma. [1]
2. Melting point (℃): -85[2]
3. Boiling point (℃): 115.8[3]
4. Relative density (water=1): 0.80 (25℃)[4]
5. Relative vapor density (air = 1): 3.5[5]
6. Saturated vapor pressure (kPa): 2.13 (20℃)[6]
7. Heat of combustion (kJ/mol): -3740[7]
8. Critical temperature (℃): 298.2[8]
9. Critical pressure (MPa): 3.27[9]
10. Octanol/water partition coefficient: 1.31[10]
11. Flash point (℃): 16 (TCC); 23.9 (OC); 23 (CC) [11]
12. Ignition temperature (℃): 449[12]
13. Explosion upper limit (%): 7.5[13]
14. Lower explosion limit (%): 1.4[14]
15. Solubility: slightly soluble in water, easily soluble in most Organic solvents. [15]
16. Refractive index (n20ºC): 1.3957
17. Refractive index (n25ºC): 1.3933
18 . Viscosity (mPa·s, 25ºC): 0.542
19. Heat of evaporation (KJ/mol): 36.47
20. Specific heat capacity (KJ/(kg·K), 20ºC, Constant pressure): 1.93
21. Electrical conductivity (S/m, 35ºC): <5.2×10-8
22. Thermal conductivity ( W/(m·K)): 0.1386
23. Body expansion coefficient (K-1, 20~30ºC): 0.00116
24. Body Expansion coefficient (K-1, 10~30ºC): 0.00112
25. Eccentricity factor: 0.389
26. Solubility parameter (J·cm-3)0.5: 17.825
27. van der Waals area (cm2·mol-1): 9.880×109
28. van der Waals volume (cm3·mol-1 ): 69.720
29. Liquid phase standard hot melt (J·mol-1·K-1): 212.4
30. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3783.57
31. Gas phase standard claimed heat (enthalpy) (kJ·mol -1):-292.46
Toxicological data
1. Irritation: Rabbit eye: 40mg, severe irritation. Rabbit transdermal: 500mg/24 hours, moderate irritation.
2. Acute toxicity: Rat oral LD50: 2080mg/kg; Rat inhalation LD50: 32720mg/m3/4H;
3. This product has strong local irritation and toxicity. It has strong toxicity and local irritation, and can cause headache, vomiting and discomfort at a concentration of 409mg/m3. The maximum allowable concentration in the workplace is 409mg/m3.
4. Acute toxicity<Under the catalysis of phosphoric acid, diacetone alcohol is dehydrated to obtain isopropylacetone. After gasification, it is mixed with hydrogen and enters the reactor. Under the catalysis of copper, the hydrogenation reaction is carried out at 170~200℃ and 0.3~1MPa, and then distilled , collect the 115.5-116.5℃ fraction (normal pressure), which is the finished product. The finished product is usually obtained by vacuum distillation at 2666~6665Pa.
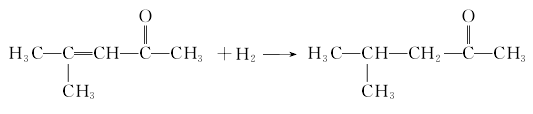
Purpose
1. This product is a medium boiling point solvent and chemical intermediate with excellent performance. Due to its stable chemical properties, it can be used as a solvent for various industrial coatings and can also be used for the production of high-end automotive coatings. Solvent for paints, inks, audio tapes, video tapes, etc., and also used as a solvent for DDT, 2,4-D, pyrethrins, penicillin, tetracycline, adhesives, and rubber glue. It is also used as mineral processing agent, grease dewaxing agent and coupler for color films. This product is an effective separating agent for some inorganic salts. It can separate plutonium from uranium, niobium from tantalum, zirconium from hafnium, etc. It also has excellent solubility for organometallic compounds. The peroxide of this product is an important initiator in the polymerization reaction of polyester resin. This product is also used as a solvent for organic synthesis and atomic absorption spectrophotometric analysis.
2. Mainly used as a solvent, it is a stable medium boiling point solvent. In addition to being widely used as a solvent for coatings, paint strippers, and various synthetic resins, it is also used as a solvent for adhesives, DDT, 2,4-D, pyrethroids, penicillin, tetracycline, rubber glue, and atomic absorption spectrophotometric analysis. . It also has excellent solubility for organometallic compounds. It is also used as mineral processing agent, grease dewaxing agent and coupler for color films. It is also an effective separating agent for some inorganic salts, which can separate plutonium from uranium, niobium from tantalum, zirconium from hafnium, etc. MIBK’s peroxide is an important initiator in the polymerization reaction of polyester resin. Domestically, it is mainly used in some special coatings and some organic synthesis (such as the synthesis of some surfactants, hydrogenation to produce MIBC, synthetic rubber antioxidants, etc.). Most applications have not yet been developed, especially in coatings and adhesives. There is still great market potential in the market. It should be noted that MIBK is irreplaceable in some application fields.
3. Used as analytical reagents, such as chromatographic analysis standard materials. Also used as solvent and extraction agent.
4. Used in the manufacture of nail polish in cosmetics. Used as a medium boiling point solvent (100~140℃) in nail polish to give the nail polish spreadability and suppress blurry feeling.
5. Used as a solvent for spray paint, nitrocellulose, certain fiber ethers, camphor, grease, natural and synthetic rubber. [25]

 微信扫一扫打赏
微信扫一扫打赏

