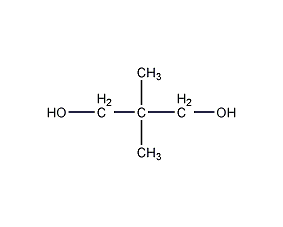
Structural formula
| Business number | 03K1 |
|---|---|
| Molecular formula | C5H12O2 |
| Molecular weight | 104.15 |
| label |
2,2-Dimethyl-1,3-propanediol, neopentyl glycol, dimethyltrimethylene glycol, Neopentyl glycol, Heat stabilizer for polyester resin, Lubricant for premium lubricants, Plasticizer |
Numbering system
CAS number:126-30-7
MDL number:MFCD00004685
EINECS number:204-781-0
RTECS number:TY5775000
BRN number:605291
PubChem number:24865974
Physical property data
1. Properties: white crystalline solid, hygroscopic.
2. Boiling point (ºC, 101.3kPa): 210
3. Melting point (ºC): 129
4. Relative density (g/mL, 25 /4ºC): 1.066
5. Relative vapor density (g/mL, air=1): Undetermined
6. Flash point (ºC, open): 129
7. Ignition point (ºC): 388
8. Heat of combustion (KJ/mol): -3100
9. Heat of fusion (KJ/mol): 21.77
10. Sublimation temperature (ºC): 128
11. Solubility: soluble in water, ethanol, ether, acetone, toluene and other solvents.
12. The standard heat of combustion (enthalpy) of the crystal phase (kJ·mol-1): -3131.3
13. The standard claim heat of the crystal phase ( Enthalpy) (kJ·mol-1): -551.2
Toxicological data
1. Acute toxicity data: Rat oral LDLo: 3200 mg/kg
Rat inhalation LCLo: 39000 ppm/6H
2. Low toxicity, irritating to skin It is of little sex, and if eaten in large quantities, it will stimulate the central nervous system and cause symptoms such as vomiting and difficulty breathing. Rat oral LD50: >6400mg/kg.
Ecological data
None
Molecular structure data
1. Molar refractive index: 28.24
2. Molar volume (cm3/mol): 106.1
3. Isotonic specific volume (90.2K ): 259.6
4. Surface tension (dyne/cm): 35.8
5. Polarizability (10-24cm3):11.19
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0
2. Hydrogen bond supply�Number: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers :None
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 44
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Uncertain atoms Number of stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is hygroscopic and has excellent solubility.
2. Low toxicity. Rat oral LD50≥6400mg/kg. Mouse oral LD503200~6400mg/kg. Little irritation to skin. However, drinking large amounts can stimulate the central nervous system, causing symptoms such as vomiting, fatigue, lethargy, difficulty breathing, tremors, kidney congestion and bleeding, liver fatty disease, urination, bronchitis and pneumonia, and even death in severe cases.
Storage method
1. Storage: Store in a ventilated and sealed place in a dry environment.
2. Packed in galvanized iron barrels, 25kg per barrel. Store in a cool, ventilated place, protected from sun, heat and moisture. Store and transport according to general chemical regulations.
Synthesis method
1. Sodium formate method Isobutyraldehyde and formaldehyde are condensed at 30-35°C, pH 9-11, and in the presence of an alkali catalyst to form hydroxyl-tert-butyraldehyde, which is reduced to neopentyl glycol with excess formaldehyde under strong alkali conditions. Formaldehyde is oxidized and reacts with alkali to form sodium formate. The reaction solution is neutralized with formic acid, distilled and dehydrated under reduced pressure, and the concentrated solution is extracted by layers. After removing sodium formate, it is cooled, crystallized, and separated to obtain the finished product.
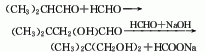
2. Hydrogenation method consists of different Butyraldehyde and formaldehyde are condensed to obtain hydroxypivalaldehyde, which is then reduced by hydrogenation.
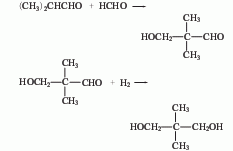
3. Preparation method:
p>
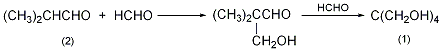
Equipped with stirrer, thermometer, drip Add 800mL of ethanol ① and 175g of potassium hydroxide (3mol) into the liquid funnel and the reaction bottle of the reflux condenser, cool in an ice-water bath, and add dropwise formaldehyde (40%, 500mL, 6.4mol) and isozoic acid. Prepare a solution of 180g (2.5mol) butyraldehyde (2) and an appropriate amount of ethanol. Control the temperature of the reaction solution at about 30°C. After the addition, continue to stir the reaction for 2 hours. Then reflux the reaction for 5 hours. Ethanol and water are recovered under reduced pressure. Cool and extract four times with diethyl ether. Distillation to recover diethyl ether. The residue is recrystallized from benzene to obtain neopentyl glycol (1). The yield is 50%. Note: ① Methanol can also be used instead of ethanol. This reaction is exothermic. Potassium formate is generated during the reaction and can be recovered. [1]
Purpose
Usage: It has a wide range of uses, mainly as a plasticizer for the production of unsaturated polyester resin; oil-free alkyd resin; polyurethane foam and elastomers, additives for high-grade lubricants and other fine chemicals. It is also an excellent solvent for the selective separation of aromatics and cycloalkyl hydrocarbons. Neopentyl glycol is water and chemical resistant and weather resistant. Its amino baking paint has good gloss retention and is non-yellowing. It can also be used as raw material for the production of polymerization inhibitors, stabilizers and pesticides. Heat stabilizer for polyester resin, lubricant for high-grade lubricants, and plasticizer.

 微信扫一扫打赏
微信扫一扫打赏

