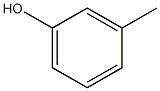
Structural formula
| Business number | 02X1 |
|---|---|
| Molecular formula | C7H8O |
| Molecular weight | 108.14 |
| label |
3-methylphenol, m-cresol, Distilled lignooleic acid, 3-Cresol, m-Hydroxytoluene, 3-Cresol, 3-Methylphenol, m-Methylphenol, 3-Hydroxytoluene, Liquid crystal materials and intermediates |
Numbering system
CAS number:108-39-4
MDL number:MFCD00002302
EINECS number:203-577-9
RTECS number:GO6125000
BRN number:506719
PubChem number:24867826
Physical property data
1. Properties: colorless to light yellow transparent liquid with aromatic odor. [1]
2. Melting point (℃): 12[2]
3. Boiling point (℃): 202.8 [3]
4. Relative density (water = 1): 1.03[4]
5. Relative vapor density (Air=1): 3.72[5]
6. Saturated vapor pressure (kPa): 0.13 (72℃)[6]
7. Heat of combustion (kJ/mol): -3680.5[7]
8. Critical temperature (℃): 432[8]
9. Critical pressure (MPa): 4.56[9]
10. Octanol/water partition coefficient: 1.96[ 10]
11. Flash point (℃): 86 (CC) [11]
12. Ignition temperature (℃) :558[12]
13. Explosion upper limit (%): 7.6[13]
14. Explosion lower limit (%) %): 1.1[14]
15. Solubility: Slightly soluble in water, miscible in ethanol, ether, sodium hydroxide aqueous solution, acetone, chloroform, etc. [15]
16. Heat of evaporation (KJ/kg, b.p.): 438.27
17. Heat of fusion (KJ/mol): 9.37
18. Specific heat capacity (KJ/(kg·K), 0~20ºC, constant pressure): 2.01
19. Electrical conductivity (S/m): 1.397×10– 8
20. Solubility (%, 25ºC, water): 2.36
21. Relative density (25℃, 4℃): 1.029
22. Refractive index at room temperature (n25): 1.5384
23. Eccentricity factor: 0.449
24. Solubility parameter (J·cm-3)0.5: 24.080
25. van der Waals area (cm2·mol-1): 8.180×1010
26. van der Waals volume (cm3·mol-1) : 65.030
27. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3765.60
28. Gas phase standard claimed heat (enthalpy) )( kJ·mol-1): -132.30
29. Gas phase standard entropy (J·mol-1·K-1 ): 365.15
30. Gas phase standard free energy of formation (kJ·mol-1): -40.1
31. Gas phase standard heat Melting (J·mol-1·K-1): 124.68
32. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -3703.89
33. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -194.01
34.Liquid�It can be separated by distillation. The 4,6-di-tert-butyl m-cresol obtained after separation can be dehydrocarbonized and decomposed into m-cresol and isobutylene by heating to 202°C in the presence of a catalyst. The m-cresol content of the obtained product can reach more than 98%. The recovery rate is about 95%. The recovery rate of isobutylene is above 95% and can be recycled. This method can be used to separate m-cresol and co-produce 2,6-di-tert-butyl p-cresol antioxidant.
Purpose
1. Mainly used as pesticide intermediates to produce the pesticides fenitrothion, fenthion, permethrin, and permethrin. It is also an intermediate for color films, resins, plasticizers and spices. Used as a disinfectant, fumigant and photographic developer.
2. Used as analytical reagents and used in organic synthesis.
3. Used as an intermediate in the synthesis of resins, color films, spices, etc. Pure m-cresol can also be used as a raw material for the organophosphorus pesticides fenitrothion, fenthion and carbamate pesticides mefeniocarb and the pyrethroid permethrin; used in the pharmaceutical industry to prepare pesticides. Ringworm powder tribromocresol; also used in the production of other fine chemicals.
4. Used as analytical reagents and in organic synthesis. [28]

 微信扫一扫打赏
微信扫一扫打赏

