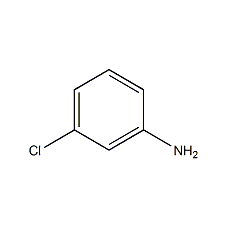
Structural formula
| Business number | 02X4 |
|---|---|
| Molecular formula | C6H6ClN |
| Molecular weight | 127.57 |
| label |
m-chloroaniline, m-Aminochlorobenzene, 3-Aminochlorobenzene, 3-Chloroaniline, 3-Chloro-Benzenamine, m-Chloroaniline, Multifunctional solvents, aromatic compounds |
Numbering system
CAS number:108-42-9
MDL number:MFCD00007765
EINECS number:203-581-0
RTECS number:BX0350000
BRN number:605969
PubChem number:24861962
Physical property data
1. Properties: colorless liquid to light amber liquid. [1]
2. Melting point (℃): -10.4[2]
3. Boiling point (℃): 230.5[3]
4. Relative density (water = 1): 1.22[4]
5. Relative vapor Density (air=1): 4.4[5]
6. Saturated vapor pressure (kPa): 0.13 (63.5℃)[6]
7. Critical pressure (MPa): 4.59[7]
8. Octanol/water partition coefficient: 1.88[8]
9. Flash point (℃): 105[9]
10. Ignition temperature (℃): 540[10 ]
11. Explosion upper limit (%): 8.8[11]
12. Explosion lower limit (%): 1.5 [12]
13. Solubility: Insoluble in water, soluble in most organic solvents. [13]
Toxicological data
1. Acute toxicity[14]
LD50: 256mg/kg (rat oral); 250mg/kg (rat transdermal)
LC50: 550mg/m3 (mouse inhalation, 4h)
2. Irritation No data yet
3. Mutagenicity[15] Microbial mutagenicity: Salmonella typhimurium Bacteria 200mg/L. Mammalian somatic mutation: hamster lung 300μg/L.
Ecological data
1. This substance may be harmful to the environment. It is recommended not to let it enter the environment.
2. Ecotoxicity[16]
LC50: 6.8mg/L (28d ) (zebrafish); 13mg/L (14d) (red killifish)
EC50: 26mg/L (48h) (green algae); 1.8mg/L (48h) (water fleas); 100mg/ L (24h) (Tetrahymena pyriformis, static)
3. Biodegradability No data available
4. Non-biodegradable Sex[17] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 5.1h (theoretical).
Molecular structure data
1. Molar refractive index: 35.38
2. Molar volume (cm3/mol): 103.6
3. Isotonic specific volume (90.2K ):268.9
4. Surface tension (dyne/cm): 45.3
5. Polarizability: 14.02
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 74.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. This product is toxic. Poisoning can occur if swallowed, inhaled or absorbed through the skin. It is hemolytic and can even cause bladder cancer. In acute poisoning, coma, cyanosis and severe shock may occur. Production equipment should be sealed and good ventilation should be maintained at the production site. Operators should wear various labor protection equipment, wash work clothes frequently, and strengthen nutrition.
2. Stability[18] Stable
3. Incompatible substances[19] Acids, acid chlorides, acid anhydrides, chloroform, strong oxidants
4. Conditions to avoid contact[20] Heating
5. Polymerization hazard[21] No polymerization
6. Decomposition products[22 ] Ammonia, hydrogen chloride
Storage method
Storage Precautions[23] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
It is obtained by reducing m-nitrochlorobenzene with iron powder in the presence of ferrous sulfate: it can also be reduced with alkali sulfide. After the reduction reaction, it is neutralized, steam distilled, and distilled under reduced pressure to obtain the finished product. The industrial product is a slightly yellow to light brown oily liquid with a content of ≥99%. Raw material consumption quota: m-nitrochlorobenzene 1300kg/t, alkali sulfide 960kg/t.
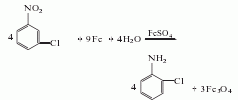
Purpose
1. Used as intermediates for dyes, pesticides and medicines. Used to prepare azo dyes, ice dye bases and printing developers, such as orange-based GC dyes. In medicine, chlorpromazine, chloroquine phosphate, etc. can be prepared. It can also be used to make pesticides, etc.
2. Important organic chemical raw materials. Widely used in dye, pesticide and pharmaceutical synthesis industries. It can be used as an intermediate for ice dye color base and developer for cotton and viscose fabric dyeing and printing. In the pharmaceutical industry, it is used to prepare chlorpromazine, chloroquine phosphate, etc.
3. Used for making azo dyes, pigments, drugs, pesticides, etc. [24]

 微信扫一扫打赏
微信扫一扫打赏

