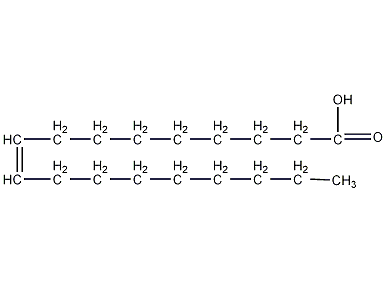
Structural formula
| Business number | 036P |
|---|---|
| Molecular formula | C18H34O2 |
| Molecular weight | 282.46 |
| label |
(9Z)-9-octadecenoic acid, red oil, animal oleic acid, cotton oleic acid, cis-9-octadecenoic acid, (9Z)-9-octadecenoic acid, (Z)-9-Octadecenoic acid, (Z)-Oleic acid, acid solvents, aliphatic compounds |
Numbering system
CAS number:112-80-1
MDL number:MFCD00064242
EINECS number:200-001-8
RTECS number:LP8925000
BRN number:1726542
PubChem number:24278605
Physical property data
1. Properties: light yellow oily liquid with an odor similar to lard. The color will gradually darken if left in the air for a long time.
2. Boiling point (ºC, 101.3kPa, decomposition): 360, 220~222ºC (933.1pa)
3. Melting point (ºC): 13~14
4. Relative density (g/mL, 20/4ºC): 0.8935
5. Relative density (g/mL, 25/4ºC): 0.845590
6. Relative density (g/mL, 90/4ºC): 0.8429
7. Relative vapor density (g/mL, air=1): 1.03
8 . Refractive index (n20ºC): 1.4582
9. Refractive index (35ºC): 1.4544
10. Viscosity (mPa·s, 20ºC): 38.80
11. Viscosity (mPa·s, 25ºC): 27.64
12. Viscosity (mPa·s, 60ºC): 9.41
13. Viscosity (mPa·s, 80ºC): 4.85
14. Flash point (ºC): >110
15. Fire point (ºC): 362.8
16. Heat of vaporization (KJ/mol, b.p.) : 167.41
17. Heat of combustion (KJ/mol, 25ºC, liquid): 11153.2
18. Specific heat capacity (KJ/(kg·K), 50ºC, constant pressure): 2.05
19. Specific heat capacity (KJ/(kg·K), 100ºC, constant pressure): 2.30
20. Specific heat capacity (KJ/(kg·K), 180ºC, constant pressure) :2.67
21. Critical pressure (MPa): 3.04
22. Conductivity (S/m, m.p.): 3×10-13
23. Conductivity (S/m, b.p.): 2.8×10-9
24. Vapor pressure (kPa, 176ºC): 0.133 p>
25. Solubility: Hardly soluble in water. Miscible with alcohol, ether, chloroform, light gasoline, etc. It is an excellent solvent for oils, fatty acids and oil-soluble substances.
26.&n��Method.
3.Hydrolyze the oil to obtain mixed fatty acids. The liquid part obtained by cold pressing is oleic acid, and the solid part saturated fatty acids are removed. Acetone can be used as a solvent to obtain pure product.
4. Adopt stearic acid co-production method. When synthesizing stearic acid, the pressed unsaturated fatty acids are further frozen and pressed to separate the saturated fatty acids and then dehydrated. Or when preparing vegetable oil, it can be obtained by saponification, acidification, separation and distillation of soap stock. .
5. Tobacco: OR, 44, FC, 9, 13, 37, 41, 44; OR, 26; FC, BU, OR, 18; BU, 9; BU, 10; FC, BU, OR, 19
Purpose
. 1. Mainly used to prepare plastic plasticizers epoxy butyl oleate and epoxy octyl oleate. Used in the wool spinning industry to prepare antistatic agents and lubricating softeners. Used in the wood industry to prepare water-repellent paraffin emulsions. Azelaic acid can be prepared through oxidation, and azelaic acid is a raw material for making polyamide resin. It can also be used to manufacture pesticide emulsifiers, printing and dyeing auxiliaries, industrial solvents, mineral flotation agents and release agents, etc. It can also be used to prepare copy paper, typing paper, ballpoint pen oil and various oleates, etc.
2.Can be used as drilling mud lubricant and anti-sticking agent. The sodium or potassium salt of oleic acid is one of the ingredients of soap. Pure sodium oleate has good detergency, can be used as a surfactant such as an emulsifier, and can be used to treat cholelithiasis. Other metal salts of oleic acid can also be used in waterproof fabrics, lubricants, polishes, etc. Its barium salt can be used as a rodenticide.
3.For biochemical analysis and gas chromatography standard materials.
4.Used to prepare plastic plasticizers, pesticide emulsifiers, lubricants, ointments, ore flotation agents, etc.
5.Used as raw material for detergents, fatty acid soap bases, cosmetics, chemical fiber oils, and textile auxiliaries. Refined oleic acid is used as raw material for plastics, engineering plastics, nylon 8 and nylon 9.
6. Used in baked goods, meat products, and condiments.

 微信扫一扫打赏
微信扫一扫打赏

