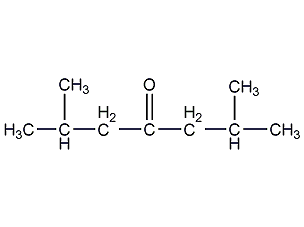
Structural formula
| Business number | 02Y0 |
|---|---|
| Molecular formula | C9H18O |
| Molecular weight | 142.24 |
| label |
diisopropylacetone, Diisobutyl(methyl)ketone, diisobutyl ketone, diisobutyl ketone, 2,6-Dimethyl-4-heptanone (diisobutyl ketone), dimethyl-tetraheptanone, 2,6-dimethyl-4-heptanone, (iso-C4H9)2CO, 2,6-Dimethyl-4-heptanone, Diisobutyl ketone, 2,6-Dimethyl-heptan-4-one, aliphatic compounds |
Numbering system
CAS number:108-83-8
MDL number:MFCD00008940
EINECS number:203-620-1
RTECS number:MJ5775000
BRN number:1743163
PubChem number:24863846
Physical property data
1. Properties: colorless oily liquid with mint aroma.
2. Relative density (g/mL, 20/4℃): 0.8060
3. Relative vapor density (g/mL, air=1): 4.9
4. Melting point (ºC): -46.0
5. Boiling point (ºC, normal pressure): 168.1
6. Refractive index (n20ºC): 1.4121
7. Viscosity (mPa·s, 0~40ºC): 1.32~0.665
8. Flash point (ºC, opening): 60
9. Autoignition point or ignition Combustion temperature (ºC): 396
10. Vapor pressure (kPa, 20ºC): 0.27
11. Heat of evaporation (KJ/mol, 168.24~86.41ºC): 39.94~46.14 ×10-3
12. Heat of formation (KJ/mol, liquid): 408.6
13. Heat of formation (KJ/mol, gas): 357.7
14. Critical temperature (ºC): 340
15. Critical pressure (KPa): 3.04
16. Specific heat capacity (KJ/(kg·K ), 15ºC, constant pressure): 2.06
17. Explosion upper limit (%, V/V): 7.1
18. Explosion lower limit (%, V/V): 0.8
19. Body expansion coefficient (K-1): 0.00102
19. Solubility: miscible with most organic solvents such as alcohols and ethers. Can dissolve cellulose acetate, cellulose nitrate, polystyrene, vinyl resin, wax, varnish, natural resin and raw rubber, etc. It dissolves 0.043% in water at 25℃; water dissolves 0.4% in 2,6-dimethyl-4-heptanone at 23℃.
20. Relative density (25℃, 4℃): 0.790040
21. Solubility parameter (J·cm-3)0.5: 16.454
22. van der Waals area (cm2·mol-1): 1.392×1010
23. van der Waals volume (cm3·mol-1): 100.400
24. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -5756.42
25. Gas phase standard claimed heat (enthalpy) (kJ·mol -1): -357.65
26. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -5705.50
27. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -408.57
28. Liquid phase standard hot melt (J·mol-1·K-1): 305.7
Toxicological data
1. Acute toxicity: rat oral LD50: 5750 mg/kg; rabbit dermal LD50: 1600mg/kg
2. Due to low vapor pressure, it is not very toxic when used industrially. It is slightly toxic, and high concentrations can cause damage to the liver and kidneys, irritate mucous membranes, and have anesthetic effects. Inhalation of vapor or repeated exposure can cause symptoms such as headache, fatigue, dizziness, and anemia, and can irritate the eyes and nose. If the human body inhales it at a concentration of 2324mg/m3 for one hour, it can cause severe poisoning. The maximum allowable concentration in the workplace is 2905mg/m3.
3. Acute toxicity:
Oral LD50 1416mg/kg(mus)
5750 mg/kg(rat)
Skin LD50 16000mg/kg(rbt)
Inhalation LCLo/4H 2000ppm/4H(rat)
Skin irritation mild 500mg(rbt)
10mg/24H(rbt)
Eye irritation mild 500mg(rbt)
Main irritant effect:
On the skin: Causes mild irritant effect.
In eyes: May cause irritation
Sensitization: No known sensitizing effects.
Ecological data
General remarks
Water hazard level 1 (German regulations) (self-assessment via list) This substance is slightly hazardous to water.
Do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems. .
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 43.69
2. Molar volume (cm3/mol): 174.9
3. Isotonic specific volume (90.2K ): 390.0
4. Surface tension (dyne/cm): 24.6
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 17.32
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 91.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidants, strong reducing agents, and strong alkalis.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, reducing agents and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Acid-catalyzed condensation of acetone produces fulone, which is then hydrogenated and reduced to diisobutyl ketone.
Refining method: Generally refined by distillation. It can also be passed through a column filled with silica gel to remove alcohol, unsaturated ketones, water, etc. and then distilled.
Purpose
This product is mainly used as an organic solvent and can also be used in organic synthesis. Can dissolve cellulose acetate, nitrocellulose, polystyrene, vinyl resin, wax, varnish, natural resin and raw rubber, etc. Due to its high boiling point and slow evaporation rate, it can be used as a solvent for nitrocellulose spray paint, vinyl paint, and other synthetic resin paints to improve their moisture-proof capabilities. It is also used as a dispersant in the manufacture of organic aerosols, a solvent for food refining, and an intermediate for certain drugs and pesticides.

 微信扫一扫打赏
微信扫一扫打赏

