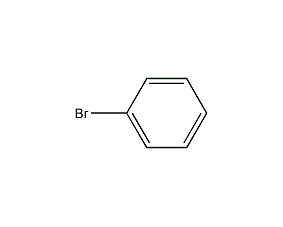
Structural formula
| Business number | 02Y3 |
|---|---|
| Molecular formula | C6H5Br |
| Molecular weight | 157 |
| label |
Bromobenzene, bromobenzene, phenyl bromide, brominated benzene, Bromobenzene, 1-Bromobenzene, Phenyl Bromide, Monobromobenzene, Pesticide intermediates; aromatic halogen derivatives |
Numbering system
CAS number:108-86-1
MDL number:MFCD00000055
EINECS number:203-623-8
RTECS number:CY9000000
BRN number:1236661
PubChem number:24891860
Physical property data
1. Properties: Colorless oily liquid with benzene odor. [1]
2. Melting point (℃): -30.7[2]
3. Boiling point (℃): 156.2[3]
4. Relative density (water = 1): 1.50[4]
5. Relative vapor Density (air=1): 5.41[5]
6. Saturated vapor pressure (kPa): 0.532 (25℃)[6]
7. Heat of combustion (kJ/mol): -3124.6[7]
8. Critical temperature (℃): 397[8]
9. Critical pressure (MPa): 4.52[9]
10. Octanol/water partition coefficient: 2.99 [10]
11. Flash point (℃): 51 (CC) [11]
12. Ignition temperature (℃) ): 565[12]
13. Explosion upper limit (%): 9.1[13]
14. Explosion lower limit (%): 1.5[14]
15. Solubility: Insoluble in water, soluble in most organic solvents such as methanol, ether, acetone, benzene, carbon tetrachloride, etc. [15]
16. Refractive index (n20ºC): 1.5601
17. Refractive index (n25ºC): 1.5576
18 . Viscosity (mPa·s, 20ºC): 1.13
19. Viscosity (mPa·s, 40ºC): 0.89
20. Viscosity (mPa·s, 100ºC): 0.52
21. Heat of evaporation (KJ/mol): 37.93
22. Heat of evaporation (KJ/kg, 25ºC): 297.7
23. Heat of fusion (KJ /mol): 10.63
24. Heat of fusion (KJ/kg, 15ºC): 67.77
25. Heat of formation (KJ/mol, liquid): 43.88
26. Heat of formation (KJ/kg, 25ºC): 682.4
27. Heat of combustion (KJ/kg): 19916.6
28. Specific heat capacity (KJ/(kg·K ), 25ºC, constant pressure): 1.0
29. Specific heat capacity (KJ/(kg·K), 29ºC, constant pressure): 0.93
30. Electrical conductivity (S/m , 25ºC): 2×10-11
31. Solubility (%, water, 30ºC): 0.0446
32. Vapor pressure (kPa,- 21.9ºC): 0.00158
33. Vapor pressure (kPa, 26.1ºC): 0.1201
34. Vapor pressure (kPa, 70ºC): 5.7328
35 .Body expansion coefficient (K-1, 20ºC): 1.000
36. Body expansion coefficient (K-1, bp.): 0.9491
37. Critical density (g·cm-3): 0.485
38. Critical volume (cm3·mol-1): 324
39. Critical compression factor: 0.263
40. Eccentricity factor: 0.251
41. Solubility parameter (J·cm-3)0.5: 19.945
42. van der Waals area (cm2·mol-1): 7.340×109
43. van der Waals volume (cm3·mol-1 ): 60.160
44. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): 105.4
45. Gas phase standard entropy (J· mol-1·K-1): 326.11
46. Gas phase standard free energy of formation (kJ·mol-1): 136.5
47. Gas phase standard hot melt (J·mol-1·K-1): 100.37
48. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): 60.9
49. Liquid phase standard entropy (J·mol-1·K-1): 219.22
50. Liquid phase standard free energy of formation (kJ·mol-1): 125.8 p>
51. Liquid phase standard hot melt (J·mol-1·K-1): 159.1
Toxicological data
1. Acute toxicity[16]
LD50: 2383mg/kg (rat oral)
LC50 : 20411mg/m3 (rat inhalation)
2. Irritation No data available
3. Subacute and chronic toxicity[17] Rats inhaled 20mg/m3 for 4 and a half months, growth inhibition, inhibition of nervous system function, and liver function disorders , sulfhydryl groups decreased in serum and liver homogenate, and serum albumin concentration decreased.
4. Mutagenicity [18] Micronucleus test: 120mg/kg in the abdominal cavity of mice (24h). Sister chromatid exchange: Hamster ovary 500mg/L. DNA repair: E. coli 250mg/L. Micronucleus test: mice were given 125mg/kg intraperitoneally (24h)
Ecological data
1. Ecotoxicity[19] LC50: 6.8mg/L (48h) (medaka)
2. Biology Degradability No information available
3. Non-biodegradability [20] In the air, when the hydroxyl radical concentration is 5.00× At 105 pieces/cm3, the degradation half-life is 21 days.
4. Bioconcentration [21] BCF: 8.8~34 (carp, exposure concentration 50ppb, exposure time 6 weeks); 12~ 33 (carp, exposure concentration 5ppb, exposure time 6 weeks)
Molecular structure data
1. Molar refractive index: 33.94
2. Molar volume (cm3/mol): 105.6
3. Isotonic specific volume (90.2K ): 257.7
4. Surface tension (dyne/cm): 35.4
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 13.45
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 46.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[22] Stable
2. Incompatible substances[23] Strong oxidizing agent
3. Polymerization hazard[24] No polymerization
4. Decomposition products [25] Hydrogen bromide
Storage method
Storage Precautions[26] Store in a cool, ventilated warehouse. The storage temperature should not exceed 37°C. Keep away from fire and heat sources. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Obtained from the reaction of benzene and bromine. First add iron powder and benzene into the reactor, slowly add bromine under stirring, and after the addition is completed, keep the reaction at 70-80°C for 1 hour. The crude product obtained is washed with water and 5% sodium hydroxide solution, left to separate, and distilled. Dry, filter, and finally fractionate under constant pressure, and take the 155-157°C fraction to obtain the finished product.
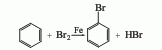
Purpose
Used as solvents, analytical reagents, lubricant additives and organic synthesis. [27]

 微信扫一扫打赏
微信扫一扫打赏

