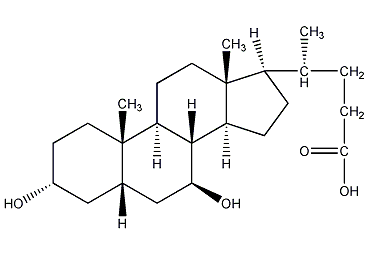
Structural formula
| Business number | 03LF |
|---|---|
| Molecular formula | C24H40O4 |
| Molecular weight | 292.58 |
| label |
3alpha,7beta-dihydroxy-5beta-cholestane-24-acid, Ursodeoxycholic acid, Ursodeoxycholic acid, Ursodeoxycholic acid, 3α,7β-Dihydroxy-5β-cholan-24-oic acid, 5β-Cholan-24-oic acid-3α,7β-diol, 7β-Hydroxylithocholic acid, UDCS, aromatic compounds |
Numbering system
CAS number:128-13-2
MDL number:MFCD00003680
EINECS number:204-879-3
RTECS number:FZ2000000
BRN number:3219888
PubChem number:24900651
Physical property data
1. Properties: white powder.
2. Melting point (℃): 200-204
3. Solubility: Easily soluble in ethanol, insoluble in chloroform; soluble in glacial acetic acid, soluble in sodium hydroxide test solution.
Toxicological data
None
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 109.65
2. Molar volume (cm3/mol): 347.8
3. Isotonic specific volume (90.2K): 905.9
4. Surface tension (dyne/cm): 46.0
5. Polarizability (10-24cm3): 43.46
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 4.9
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 4
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 77.8
7. Number of heavy atoms: 28
8. Surface charge: 0
9. Complexity: 605
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 10
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It has the effects of dissolving gallstones, inhibiting blood cholesterol deposition, calming the liver, promoting choleretics, and detoxifying. Its sodium salt is stronger than other bile acids in promoting liver glycogen storage, fatty acid hydrolysis and hypoglycemic effects.
Storage method
Storage Shade from light and stored in an airtight container.
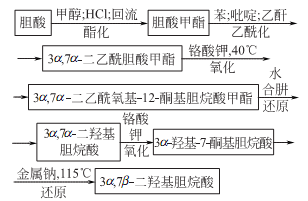
Synthesis method
Purpose
Gallstone dissolving medicine.

 微信扫一扫打赏
微信扫一扫打赏

