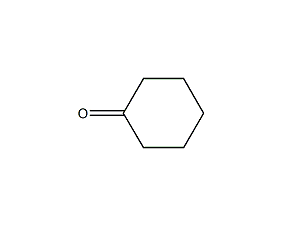
Structural formula
| Business number | 02YA |
|---|---|
| Molecular formula | C6H10O |
| Molecular weight | 98.14 |
| label |
Andone, Ketohexamethylene, Pimelic ketone, Anone, degreasing agent, adhesive, paint stripper, matting agent for silk, Raw materials and intermediates used in ink |
Numbering system
CAS number:108-94-1
MDL number:MFCD00001625
EINECS number:203-631-1
RTECS number:GW1050000
BRN number:385735
PubChem number:24857528
Physical property data
1. Properties: colorless or light yellow transparent oily liquid with a strong pungent odor. [1]
2. Melting point (℃): -32.1[2]
3. Boiling point (℃): 136.9~155.6[3]
4. Relative density (water=1): 0.95[4]
5. Relative vapor density (air=1): 3.4[5]
6. Saturated vapor pressure (kPa): 0.5 (20℃)[6]
7. Heat of combustion (kJ/mol): -3521.3[7]
8. Critical temperature (℃): 356[ 8]
9. Critical pressure (MPa): 3.8[9]
10. Octanol/water partition coefficient: 0.81[10]
11. Flash point (℃): 44 (CC) [11]
12. Ignition temperature (℃): 420[12]
13. Explosion upper limit (%): 9.4[13]
14. Lower explosion limit (%): 1.1[14]
15. Solubility: Slightly soluble in water, miscible in most organic solvents such as ethanol, ether, benzene, acetone and so on. [15]
16. Heat of evaporation (KJ/mol, 29.21ºC): 44.88
17. Heat of evaporation (KJ/mol, b.p.): 40.28
18. Heat of formation (KJ/mol, 25ºC, liquid): -271.47
19. Specific heat capacity (KJ/(kg·K), 15~18ºC, constant pressure) : 1.81
20. Electrical conductivity (S/m, 35ºC): 5×10-8
21. Thermal conductivity (W/(m ·K)): 0.1378
22. Solubility (%, 20ºC, water): 2.3
23. Volume expansion coefficient (K-1): 0.00091
24. Relative density (25℃, 4℃): 0.9411
25. Refractive index at room temperature (n25): 1.4485
26. Eccentricity factor: 0.450
27. Solubility parameter (J·cm-3)0.5: 21.143
28. van der Waals area (cm2·mol-1): 8.350×109
29 .van der Waals volume (cm3·mol-1): 62.850
30. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3564.1
31. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -226.1
32. Gas phase standard entropy (J·mol-1·K-1): 330.5
33. Gas phase standard free energy of formation (kJ ·mol-1): -89.2
34. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -3519.0
35. Liquid phase standard claims heat (enthalpy) (kJ·mol-1</s��Cyclohexanone will be further oxidized to adipic acid. ②The azeotropic point of cyclohexanone and water is 95°C. ③The solubility of cyclohexanone in water is 2.4g at 31°C. If organic solvent extraction is used instead, the yield will be slightly improved. [31]
7. Preparation method:
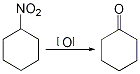
In a reaction flask equipped with magnetic stirring, add 1 mmol of nitrocyclohexane (2) and 6 mL of methanol. Add 8 mL (0.5 mol/L) Na2PO4 dissolved in 1 mol/L sodium hydroxide solution while stirring. After 1 hour, add 1 mmol potassium hydrogen persulfate dissolved in 3 mL water. The reaction was stirred at room temperature for 1 hour, and then acidified with 10% hydrochloric acid. Extract with dichloromethane, wash with saturated brine, dry with anhydrous sodium sulfate, and concentrate to obtain cyclohexanone ① (1), with a yield of 81%. Note: ① The following compounds can be synthesized using similar methods (Table I-7-10, page 308). [32]
Purpose
1. This product is the main industrial solvent. Used in paints containing nitrocellulose, vinyl chloride polymers and their copolymers, and methacrylate polymers. It can be used as a solvent for polyvinyl chloride, vinyl chloride copolymers, and methacrylate polymers. As an excellent solvent for pesticides, it is used in organophosphorus pesticides and many similar products. Used as a solvent for dyes, a viscous solvent for piston aviation lubricants, and a solvent for esters, waxes and rubbers. This product is the main intermediate for the manufacture of adipic acid and caprolactam, and the main raw material for the manufacture of antioxidant 4010. It is used in medicine to produce hydrocortisone, prednisone acetate and progesterone. It is also used as a leveling agent for dyed and matted yarns, a deesterifying agent for polishing metal, and a defilming, stain-removing and stain-removing agent for wood paint.
2. It mainly uses ε-caprolactam and adipic acid as raw materials for making nylon, so it occupies an important position in industry. It has excellent dissolving ability for various organic substances. It is especially used as a solvent for nitrocellulose spray paint. It can improve the moisture resistance, ductility and adhesion of the paint, making the coating smooth and beautiful. The nitrocellulose solution of cyclohexanone has low viscosity and is easily mixed with resins or greases. In addition, it can be used as a general solvent and adhesive for synthetic resins such as polyvinyl chloride and methyl methacrylate. It can dissolve alkaline dyes and can be used for wood dyeing. It can also be used as a solvent for DDT, organophosphorus pesticides and other solvents. Others can also be used as paint removers, degreasers for textiles, leather and metals, matting agents for silk, printing inks, etc.
3. Used as analytical reagents, such as gas chromatography stationary solutions, chromatographic analysis standard materials, and organic solvents. Also used in organic synthesis.
4. Cosmetic solvents. Mainly used as a high boiling point solvent for cosmetics such as nail polish. It is usually formulated as a mixed solvent with low boiling point solvents and medium boiling point solvents to obtain suitable evaporation speed and viscosity.
5. Mainly used to manufacture caprolactam and adipic acid, and is also an excellent solvent. [30]

 微信扫一扫打赏
微信扫一扫打赏

