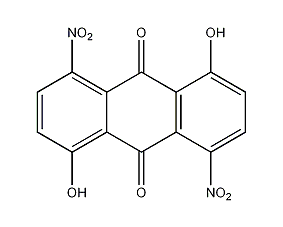
Structural formula
| Business number | 03LT |
|---|---|
| Molecular formula | C14H6N2O8 |
| Molecular weight | 330.21 |
| label |
1,5-dihydroxy-4,8-dinitroquinoneanthracene, 1,5-dihydroxy-4,8-dinitro-10-anthracenedione, 1,5-dihydroxy-4,8-dinitroanthracene-9,10-dione, 1,5-Dihydroxy-4,8-dinitroanthrachinon, 4,8-Dinitroanthrarufin, aromatic compounds |
Numbering system
CAS number:128-91-6
MDL number:MFCD00035827
EINECS number:None
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
None
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 75.52
2. Molar volume (cm3/mol): 179.6
3. Isotonic specific volume (90.2K ): 579.4
4. Surface tension (dyne/cm): 108.2
5. Polarizability (10-24cm3): 29.93
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.9
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 8
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 10
6. Topological molecule polar surface area 166
7. Number of heavy atoms: 24
8. Surface charge: 0
9. Complexity: 545
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Has a pungent odor.
Storage method
Sealed iron drum packaging
Synthesis method
It is produced by the interaction between 1,5-dichloroanthraquinone and phenol, followed by nitrification and hydrolysis. First add sodium hydroxide and phenol into the reaction kettle, and heat to 130~150°C to completely dissolve them. Cool to 120°C, add 1,5-dichloroanthraquinone, react at 145~155°C to the end point, and obtain 1,5-diphenoxyanthraquinone through distillation, filtration, water washing and drying. Then, add mixed acid and 1,5-diphenoxyanthraquinone into the nitrating kettle and perform nitration at 40°C to obtain 1,5-(2,4-dinitrophenoxy)-4,8-dinitroanthracene. Quinone. Add water and the nitrate obtained above to the hydrolysis kettle, stir evenly, adjust the volume, add 30% solution, raise the temperature to 95°C, keep it warm for 30 minutes, cool it below 50°C, filter, and wash with 3% sodium hydroxide solution until washed. No precipitation occurs after liquid acid precipitation. The filter cake is the finished product 1,5-dihydroxy-4,8-dinitroanthraquinone. The filtrate is subjected to acid precipitation and filtration to obtain 2,4-dinitrophenol.
Purpose
Dye intermediates. Used to manufacture disperse blue 2BLN dye, etc.

 微信扫一扫打赏
微信扫一扫打赏

