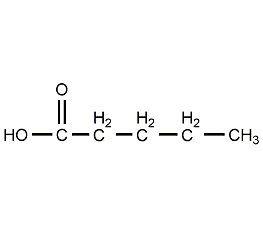
Structural formula
| Business number | 02Z3 |
|---|---|
| Molecular formula | C5H10O2 |
| Molecular weight | 102.13 |
| label |
Valeric acid, It is crossing the heart to eliminate oxalic acid, (n-)valeric acid, Propylacetic acid, n-Pentanoic acid, Propylacetic acid, Pentanoic acid, acid solvents, aliphatic compounds |
Numbering system
CAS number:109-52-4
MDL number:MFCD00004413
EINECS number:203-677-2
RTECS number:YV6100000
BRN number:969454
PubChem ID:None
Physical property data
1. Properties: Colorless liquid with an unpleasant pungent odor.
2. Boiling point (ºC, 101.3kPa): 186, 96ºC (3066pa)
3. Melting point (ºC): -34
4. Relative density (g/mL, 20/4ºC): 0.9339
5. Relative density (g/mL, 25/4ºC): 0.9345
6. Relative vapor density (g/mL, Air = 1): 3.5
7. Refractive index (n20ºC): 1.4085
8. Refractive index (n25ºC): 1.4060
9. Viscosity (mPa ·s, 15ºC): 2.359
10. Viscosity (mPa·s, 30ºC): 1.774
11. Flash point (ºC, opening): 96
12. Heat of fusion (KJ/mol): 14.17
13. Heat of evaporation (KJ/mol, 25ºC): 69.33
14. Heat of evaporation (KJ/mol, b.p.) : 44.09
15. Heat of combustion (KJ/mol, 25ºC, liquid): -2793.85
16. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 2.06
17. Critical temperature (ºC): 378
18. Critical pressure (MPa): 3.63
19. Vapor pressure (kPa, 25ºC): 0.019
20. Dissociation constant (25ºC, water): 1.38×10-5
21. Solubility: Miscible with ethanol and ether. It dissolves 2.4% in water at 20℃; water dissolves 13.0% in valeric acid.
22. Critical density (g·cm-3): 0.295
23. Critical volume (cm3·mol -1): 346
24. Critical compression factor: 0.237
25. Eccentricity factor: 0.627
26. Solubility Parameter (J·cm-3)0.5: 23.458
27. van der Waals area (cm2·mol -1): 9.230×109
28. van der Waals volume (cm3·mol-1): 63.880
29. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -2905.45
30. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -491.9
31. The gas phase standard entropy (J·mol-1·K-1): 439.82
32. Gas phase standard free energy of formation (kJ·mol-1): -357.0
33. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2837.25
34. Liquid phase standard claimed heat (Enthalpy) (kJ·mol-1): -559.44
35. Liquid phase standard entropy (J·mol-1·K-1): 259.83
36. Liquid phase standard free energy of formation (kJ·mol-1): -371.71
37 . Liquid phase standard hot melt (J·mol-1·K-1): 207.4
Toxicological data
1. Acute toxicity: Mouse oral LD50: 600mg/kg; Mouse inhalation LC50: 4100mg/m3/2H; Mouse abdominal LD50: 3590mg/kg; Mouse Subcutaneous LD50: 3590mg/kg; intravenous LD50 in mice: 1290mg/kg;
2. Concentrated acid has strong irritation to the skin. When rats are inhaled and poisoned, they may develop conjunctivitis, exercise excitement, etc.
Ecological data
This substance is harmful to the environment and can cause pollution to water bodies and the atmosphere. Organic acids can easily form acid rain during atmospheric chemistry and atmospheric physical changes. Therefore, when the pH value drops below 5, it will cause serious harm to animals and plants, and the reproduction and development of fish will be seriously affected. Metals in the soil and water sediments in the watershed can be dissolved into the water and poison the fish. Acidification of water bodies will also lead to changes in the composition and structure of aquatic organisms. Acid-resistant algae and fungi will increase, while root plants, bacteria and vertebrates will decrease, and the decomposition rate of organic matter will decrease. Acidification will seriously lead to the reduction or death of fish in lakes and rivers.
Molecular structure data
1. Molar refractive index: 26.77
2. Molar volume (cm3/mol): 105.6
3. Isotonic specific volume (90.2K ): 252.8
4. Surface tension (dyne/cm): 32.7
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 10.61
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.4
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 37.3
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 59.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants, reducing agents, and alkalis.
2.This product has low toxicity. The LD50 of intravenous injection in mice is (1290±53) mg/kg. For protective measures, see n-butyric acid.
3. Exists in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves, and smoke.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, reducing agents, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. Packed in stainless steel drums or plastic drums, net weight 100kg or 200kg. Store and transport according to general chemical regulations.
Synthesis method
1. n-pentanol is obtained by electrolytic oxidation. Formic acid can also be reacted with 1-butene to form n-valeric acid.
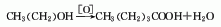
Refining method: remove water by distillation, When the temperature reaches 183°C, add water and solid potassium permanganate to reflux and continue distillation. It can also be refined by distillation under reduced pressure.
2. Tobacco: OR, 44, 49; BU, 56; OR, 26; FC, 9, 18, 40; BU, 9.
3. Preparation method:
In a reaction bottle equipped with a stirrer, thermometer and dropping funnel, add 120g (0.76mol) of finely ground potassium permanganate and water. 50 mL, 21.5 g (0.53 mol) of sodium hydroxide, slowly add 50 g (0.57 mol) of pentanol (2) dropwise within 1.5 h while stirring, be careful to add about 800 g of crushed ice to the reaction bottle at the same time to keep the temperature of the reaction solution at Between 18~24℃. After the addition was completed, the reaction was continued to stir for 1 hour, and then left at room temperature for 12 hours. Suction filtration. The filter cake is washed with hot water. Combine the filtrate and washing liquid and concentrate under reduced pressure to 150 mL. After cooling, use 130 g of cold 50% sulfuric acid to make it acidic to Congo red test paper. The organic layer was separated, and the aqueous layer was extracted with carbon tetrachloride twice, 20 mL each time. The organic layers were combined and dried over anhydrous sodium sulfate. After evaporating the solvent, the fractions at 184-187°C were collected to obtain 41g of valeric acid (1) with a yield of 70.8%. [1]
4. Preparation method:
In a reaction bottle equipped with a stirrer and a reflux condenser, Add 100g (1.2mol) of valeronitrile (2), 250mL of water, and 92g of sodium hydroxide. Heat and reflux with stirring until the nitrile layer disappears, about 5 to 10 hours. Then add 100 mL of water, and slowly add 50% sulfuric acid while cooling to make the reaction solution acidic. The upper organic layer was separated, dried over anhydrous sodium sulfate, and distilled. The fractions at 183-184°C were collected to obtain 82 g of valeric acid ① (1), with a yield of 67%. Note: ① Extract the water layer with ether and distill to obtain about 5g of valeric acid. [2]
5. Preparation method:
Refer to the above preparation method of 2-methylbutyric acid, use magnesium 12.2g of crumbs (0.51mol), one grain of iodine, 69g of n-butyl bromide (2) (54mL, 0.5mol), 250mL of anhydrous ether. First prepare the Grigard reagent, and then react with 125g of dry ice. The treatment method is the same as above. Collect 182~185℃ From the fraction, 25g of valeric acid (1) was obtained, with a yield of 49%. [3]
Purpose
Mainly used to produce valerate, a raw material for spices. Raw materials for the estrogen drug estradiol valerate and disinfectants. Used in the manufacture of spices, condiments, medicines, plasticizers, etc.
From the fraction between 184 and 187°C, 41g of valeric acid (1) was obtained, with a yield of 70.8%. [1]
4. Preparation method:
In a reaction bottle equipped with a stirrer and a reflux condenser, Add 100g (1.2mol) of valeronitrile (2), 250mL of water, and 92g of sodium hydroxide. Heat and reflux with stirring until the nitrile layer disappears, about 5 to 10 hours. Then add 100 mL of water, and slowly add 50% sulfuric acid while cooling to make the reaction solution acidic. The upper organic layer was separated, dried over anhydrous sodium sulfate, and distilled. The fractions at 183-184°C were collected to obtain 82 g of valeric acid ① (1), with a yield of 67%. Note: ① Extract the water layer with ether and distill to obtain about 5g of valeric acid. [2]
5. Preparation method:
Refer to the above preparation method of 2-methylbutyric acid, use magnesium 12.2g of crumbs (0.51mol), one grain of iodine, 69g of n-butyl bromide (2) (54mL, 0.5mol), 250mL of anhydrous ether. First prepare the Grigard reagent, and then react with 125g of dry ice. The treatment method is the same as above. Collect 182~185℃ From the fraction, 25g of valeric acid (1) was obtained, with a yield of 49%. [3]
Purpose
Mainly used to produce valerate, a raw material for spices. Raw materials for the estrogen drug estradiol valerate and disinfectants. Used in the manufacture of spices, condiments, medicines, plasticizers, etc.

 微信扫一扫打赏
微信扫一扫打赏

