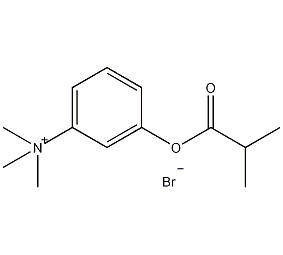
Structural formula
| Business number | 037E |
|---|---|
| Molecular formula | C12H19BrN2O2 |
| Molecular weight | 303.20 |
| label |
Prussian Ming, neostigmine bromide, (3-Dimethylcarbamoyloxyphenyl)trimethylammonium bromide, 3-(N,N-Dimethylcarbamoyloxy)-N,N,N,-trimethylanilinium bromide, aromatic compounds |
Numbering system
CAS number:114-80-7
MDL number:MFCD00011795
EINECS number:204-054-8
RTECS number:BR3150000
BRN number:None
PubChem number:24277854
Physical property data
1. Characteristics: White crystalline powder. Odorless and non-bitter.
2. Density (g/mL, 25℃): Undetermined
3. Relative vapor density (g/mL,air =1): Undetermined
4. Melting point (ºC):167℃(decomposed)
5. Boiling point (ºC,normal pressure): Undetermined
6. Boiling point (ºC,KPa):Undetermined
7. Refractive index (n20/D):Undetermined
8. Flashpoint (ºC): Undetermined
9. Specific optical rotation (ºC): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,38ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil-water (octanol/water) partition coefficient Log value of : Undetermined
17. Explosion limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility:Easily soluble in water (1gThis product is soluble in 1mlwater), ethanol, and chloroform , almost insoluble in ether.
Toxicological data
1. Acute toxicity: Rat oral LD5O: 51mg/kg
Rat transperitonealLDLO:835ug/kg
Rat subcutaneously LD5O:370ug/kg
Rat intravenous LD5O: 165ug/kg
Orally administered to miceLD5O:7mg/kg
Mouse transperitonealLD5O:610ug/kg
Mouse subcutaneouslyLD5O:130ug/kg
Cat via mouth LD5O:7449ug/kg
Cat Meridian VeinLD5O:171ug/kg
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 29.5
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 246
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
None yet
Storage method
None yet
Synthesis method
Meta-dimethylaminophenol (see 15070) and dimethylcarbamoyl chloride via ester The neostigmine base is obtained by chemical reaction, and then the quaternary ammonium salt is reacted with methyl bromide to obtain neostigmine bromide. For example, by reacting the neostigmine base with dimethyl sulfate to form a quaternary ammonium salt, neostigmine methyl sulfate ([51-60-5]).
Purpose
This product has a reversible cholinesterase inhibitory effect, causing acetylcholine to not be enzymatically decomposed and existing in cholinergic nerve endings for a long time, which can cause Exciting smoothing machine and skeletal muscle; strong effect on skeletal muscles, small miotic force; mostly used for myasthenia gravis, postoperative abdominal distension and urinary retention, and can also be used for supraventricular paroxysmal tachycardia, and Detoxification of tubocurarine overdose. Neostigmine methyl sulfate is for injection.
N>Rat intravenous LD5O:165ug/kg
Orally administered to miceLD5O:7mg/kg
Mouse transperitonealLD5O:610ug/kg
Mouse subcutaneouslyLD5O:130ug/kg
Cat via mouth LD5O:7449ug/kg
Cat Meridian VeinLD5O:171ug/kg
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 29.5
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 246
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
None yet
Storage method
None yet
Synthesis method
Meta-dimethylaminophenol (see 15070) and dimethylcarbamoyl chloride via ester The neostigmine base is obtained by chemical reaction, and then the quaternary ammonium salt is reacted with methyl bromide to obtain neostigmine bromide. For example, by reacting the neostigmine base with dimethyl sulfate to form a quaternary ammonium salt, neostigmine methyl sulfate ([51-60-5]).
Purpose
This product has a reversible cholinesterase inhibitory effect, causing acetylcholine to not be enzymatically decomposed and existing in cholinergic nerve endings for a long time, which can cause Exciting smoothing machine and skeletal muscle; strong effect on skeletal muscles, small miotic force; mostly used for myasthenia gravis, postoperative abdominal distension and urinary retention, and can also be used for supraventricular paroxysmal tachycardia, and Detoxification of tubocurarine overdose. Neostigmine methyl sulfate is for injection.

 微信扫一扫打赏
微信扫一扫打赏

