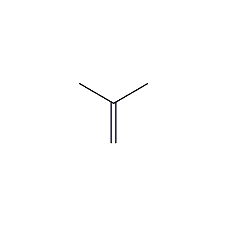
Structural formula
| Business number | 037L |
|---|---|
| Molecular formula | C4H8 |
| Molecular weight | 56.10 |
| label |
2-Methylpropene, 2-Methyl-1-propene, 1,1-dimethylethylene, 1,1-Dimethylethylene, 2-Methyl-1-propen, Isobutene, 2-Methylpropene |
Numbering system
CAS number:115-11-7
MDL number:MFCD00008898
EINECS number:204-066-3
RTECS number:UD0890000
BRN number:773645
PubChem number:24857786
Physical property data
1.Character: colorless gas[9]
2. Melting point (℃): -140.3[10]
3. Boiling point (℃): -6.9[11]
4. Relative density (water=1): 0.6 (20℃)[12]
5. Relative vapor density (air=1): 1.94[13]
6. Saturated vapor pressure (kPa): 307 (25℃)[14]
7. Heat of combustion (kJ/mol): -2866.3[15]
8. Critical temperature (℃): 144.9[16]
9. Critical pressure (MPa): 3.99[17]
10. Octanol/water partition coefficient: 2.34[18]
11. Flash point (℃): -77[19]
12. Ignition temperature (℃): 465[20]
13. Explosion upper limit (%): 9.6[21] sup>
14. Lower explosion limit (%): 1.8[22]
15. Solubility: insoluble in water, easily soluble in ethanol and ether and most organic solvents. [23]
16. Solubility parameter (J·cm-3)0.5:14.955
17.van der Waals area (cm2·mol-1): 6.710×109
18. van der Waals volume (cm3·mol-1): 44.290
19. Eccentricity factor: 0.189
20. Critical compression factor: 0.2749
21. Critical density (g·cm-3): 0.235
22. Critical volume (cm 3·mol-1): 238.8
23. Liquid phase standard hot melt (J·mol-1·K-1): 131.0
24. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2721.0
25. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -37.5
26. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -2700.4
27. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -16.9
28. Gas phase standard entropy (J·mol-1·K-1): 293.20
29. Gas phase standard formation free energy (kJ· mol-1): 58.4
30. Gas phase standard hot melt (J·mol-1·K-1 ): 88.09
Toxicological data
1. Acute toxicity[24] LC50: 620000mg/m3 (rat inhalation, 4h)
2. Irritation No information available
Ecological data
1. Ecotoxicity No information available
2., under the condition of pressure 0.29-0.39MPa, it is dehydrated into isobutylene, the single-pass conversion rate is 84%-92%, and then through distillation and purification, more than 99.9% isobutylene can be obtained. This method avoids serious corrosion by sulfuric acid, but the isobutylene content in the extract is high (up to 2%-3%), which affects its yield.
3. Methyl tert-butyl ether method: Methyl tert-butyl ether is decomposed into isobutylene and methanol in contact with an acidic catalyst at high temperature. The decomposition reaction conditions vary with different catalysts, generally the temperature is 150-300°C, the pressure is 0.1-0.98MPa, and the liquid volume hourly space velocity is 1-5h-1.
4. Isobutane dehydrogenation method: Using isobutane as raw material, catalytic dehydrogenation to produce isobutylene has the technology of Olegex of the American UOP Company.
![]()
The company uses pt/Al2O2 catalyst And the same moving bed reaction-regeneration dual-vessel reaction system as the continuous reforming; reaction temperature 620-650°C and pressure 0.2-0.25MPa; under this process condition, the single-pass isobutane dehydrogenation rate is 40%-45% (mass), the yield of isobutylene is 89% (mass), and the yield of hydrogen is 2.5%-3%. Under strict control of raw material impurities, the catalyst life can reach 2.5 years.
5. Use industrial isobutylene as raw material (isobutylene 99%), which can be purified by drying, dehydration and distillation.
6. (1) Liquid phase dehydration: Install a thermometer, a reflux condenser and a dropping funnel on a 1-liter three-neck flask. The top of the reflux condenser is connected to a downward water vapor condensation tube through a curved glass tube. The latter is then connected to the suction filter bottle in the ice bath, and the side pipe of the suction filter bottle is connected to the 0°C cold trap. Put 222 grams of tert-butyl alcohol and 15 ml of concentrated sulfur in a three-necked flask. Boil until gas evolution stops. Obtain 82% crude product. If more sulfuric acid is used, the yield will decrease; if too little, the dehydration will take a long time to complete. The crude product is distilled through a fractionating column with a reflux ratio of 15:1, and the middle fraction is collected.
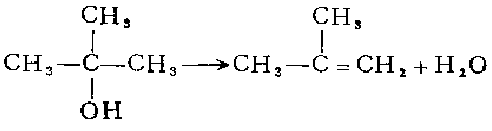
(2) Vapor phase dehydration: tert-butyl alcohol Pass through a 50 cm long, 2 cm diameter glass tube filled with 6 to 8 mesh alumina at a rate of 75 g/hour, and maintain the temperature at 375-425°C. Obtain 100% pure isobutylene.
Purpose
1. Industrially, high-concentration isobutylene is mainly used to produce polyisobutylene and copolymerize with isoprene to produce butyl rubber. The alkylation reaction between isobutylene and isobutane can produce high-octane alkylated gasoline. The methyl tert-butyl ether obtained by reacting with methanol is an excellent gasoline additive. It is also suitable for use as raw material for alkylation of aromatic hydrocarbons, or fine chemicals produced through oxidation, ammoniation and other operations.
2. High-purity isobutylene is mainly used as standard gas and for preparing special standard mixed gas.
3. Main monomer used in the production of polyisobutylene, isoprene rubber, isobutylene rubber, butyl rubber and synthetic isoprene. It is also used in the manufacture of catalysts, antioxidants, pesticides, medicines, spices, gasoline additives and lubricants.
4. Isobutylene is a good protective reagent for carboxyl and hydroxyl groups. It can undergo photochemical cycloaddition reaction, acid-catalyzed cycloaddition reaction with enone, and can also undergo alkylation reaction and carbene reaction.
Protection of carboxyl groups Isobutylene is widely used to protect carboxylic acids to generate the corresponding tert-butyl ester [1], fatty acids, aromatic acids and nitrogen-protected Acids such as amino acids can be protected by isobutylene (Formula 1)[2]. The sterically hindered tert-butyl ester is difficult to undergo saponification reaction, but can undergo hydrolysis under acid catalysis.

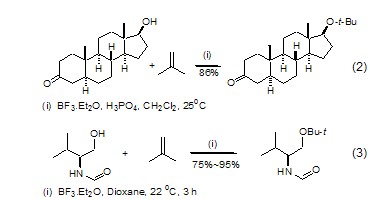
Isobutylene can react with a A series of reactions between alcohols and phenols yield the corresponding tert-butyl ethers. Tert-butyl ether is stable to most reagents but will decompose when exposed to strong acids. Propargyl alcohols, sterol alcohols and phenols can all be protected by isobutylene, and it can also protect derivatives of valine and serine and the hydroxyl group in tyrosine (Formula 2, Formula 3)[3,4].
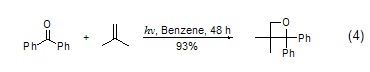
Under light conditions Cycloaddition reactionIsobutene can be widely used to perform cycloaddition with enone, and weakly protonated isobutene often undergoes stereoselective cycloaddition. Cyclohexenone, cyclopentenone and enones with functional groups can all undergo cycloaddition with isobutylene. The Paterno-Buchi cycloaddition of isobutylene can produce various alkylene oxides (Formula 4)[5].
Ene-forming reaction In the presence of Lewis acid, isobutylene can react with various alkoxy aldehydes, dialkylamine aldehydes, halogenated aldehydes and vinyl sulfoxides to obtain ene (formula 5)[6]. Chiral organoaluminum and organotitanium reagents can obtain isomerized products in high yields in the reaction of isobutylene and active aldehydes.
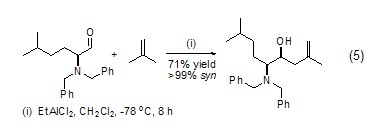
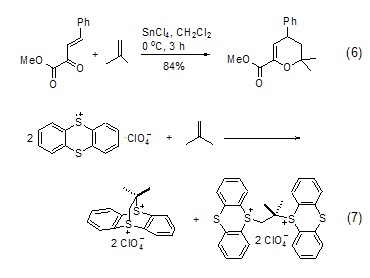
Other acid-catalyzed cycloadditions Isobutylene can obtain pyran salts and alkylene oxides through the [2+2] cycloaddition reaction under the catalysis of boron trifluoride, and through Diels-Alder reaction can also obtain 3,4-dihydropyran (formula 6)[7]. Isobutylene can also undergo cycloaddition reaction with perchlorate of thianthrene (Formula 7)[8].
5. Used to make synthetic rubber and as organic chemical raw materials. [33]
The perchlorate of �� undergoes cycloaddition reaction (Formula 7)[8].

5. Used to make synthetic rubber and as organic chemical raw materials. [33]

 微信扫一扫打赏
微信扫一扫打赏

