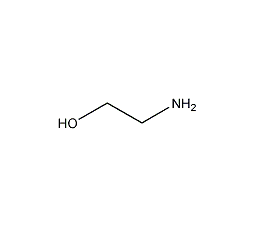
Structural formula
| Business number | 03U0 |
|---|---|
| Molecular formula | C2H7NO |
| Molecular weight | 61.08 |
| label |
2-Hydroxyethylamine, 2-aminoethanol, monoethanolamine, monoethanolamine, 1-aminoethanol, 2-aminoethanol, Aminoethanol, Acetaldehyde ammonia ethanolamine, cholamine, B-hydroxyethylamine, 2-Aminoethanol, Monoethanolamine, MEA |
Numbering system
CAS number:141-43-5
MDL number:MFCD00008183
EINECS number:205-483-3
RTECS number:KJ5775000
BRN number:505944
PubChem number:24865720
Physical property data
1. Properties: Colorless and transparent viscous liquid with hygroscopicity and ammonia odor.
2. Boiling point (ºC, 101.3kPa): 170.3
3. Melting point (ºC): 10.53
4. Relative density (g/mL, 20 /4ºC): 1.109
5. Relative density (g/mL, 20/20ºC): 1.0179
6. Relative vapor density (g/mL, air=1): 2.11
7. Refractive index (20ºC): 1.4539
8. Viscosity (mPa·s, 15ºC): 30.855
9. Viscosity (mPa·s, 30ºC): 13.9
10. Viscosity (mPa·s, 90ºC): 2.3
11. Flash point (ºC, opening): 93
12. Heat of evaporation (KJ/mol, b.p.): 49.86
13. Heat of fusion (KJ/mol): 20.515
14. Heat of combustion (KJ/mol, 25ºC): 924.99
15. Specific heat capacity (KJ/(kg·K), 30ºC, constant pressure): 2.78
16. Critical temperature (ºC): 44.1
17. Volume expansion coefficient (K-1, 20ºC): 0.00077
18. Vapor pressure (kPa, 60ºC): 0.8
19. Solubility: Energy Miscible with water, ethanol and acetone, slightly soluble in ether and carbon tetrachloride. At 25°C, the solubility in benzene is 1.4%, the solubility in ether is 2.1%, and the solubility in carbon tetrachloride is 0.2%
Toxicological data
1. Reproductive toxicity: Rat oral TDLo: 500 mg/kg; Rat oral TDLo: 4500 mg/kg;
Rat transdermal TDLo�81051506261.gif” alt=”” />
3. It is formed by the condensation of ethylene oxide and ammonia at 30~40℃ and 70.1~304kPa. It is the product of mono, di and triethanolamine. The mixed liquid is dehydrated and concentrated at 90-120°C, and then distilled under reduced pressure in a rectification tower, and the 168-174°C fraction is intercepted.
Refining method: Impurities that are easy to contain include water and ethylene glycol. Alcohol, diethanolamine, triethanolamine, etc. Part of the decomposition occurs during distillation under normal pressure, and it can be repeatedly fractionated and refined under reduced pressure (about 0.667KPa). Pay attention to prevent the absorption of carbon dioxide during fractionation. The distillate is washed with ether, and the ethanol is recrystallized. The pure product can be obtained.
4.The preparation of ethanolamine adopts the ethylene oxide amination process, which can be divided into two methods: continuous and batch. Three products, monoethanolamine, diethanolamine and triethanolamine, can be obtained. The output of the three products is usually adjusted by changing the ratio of the amounts of ammonia and ethylene oxide. In the continuous production process, the raw materials ammonia and epoxy After the ethane reacts in the tubular reactor, it then goes through the deamination tower, dehydration tower and distillation tower for deamination, dehydration and vacuum distillation to obtain monoethanolamine, diethanolamine and triethanolamine finished products respectively.
Purpose
1. Used as chemical reagents, solvents, emulsifiers, rubber accelerators, corrosion inhibitors, etc. Used as gas chromatography stationary liquid, GB 2760-96 stipulates that it is allowed to be used as a processing aid for the food industry. Used to remove acidic gases from natural gas and petroleum gas, and to manufacture nonionic detergents, emulsifiers, etc.
Monoethanolamine is mainly used as a plasticizer, vulcanizing agent, accelerator and foaming agent for synthetic resins and rubbers, as well as an intermediate for pesticides, medicines and dyes. It is also a raw material for synthetic detergents and emulsifiers for cosmetics. In the textile industry, it is used as a whitening agent, antistatic agent, anti-moth agent and detergent for printing and dyeing. It can also be used as carbon dioxide absorbent, ink additive, and petroleum additive. Monoethanolamine is widely used as a purification liquid to extract acidic components from various gases (such as natural gas). Piperazine hexahydrate can be obtained by cyclization and neutralization of monoethanolamine hydrochloride. Monoethanolamine hydrochloride is chlorinated with thionyl chloride and then replaced by sodium thiosulfate to produce β-aminoethyl thiosulfate. This is a dye intermediate used to produce polycondensation emerald blue 13G. The reaction of monoethanolamine with carbon disulfide can produce the intermediate sulfothiazoline which is used in the rubber and pharmaceutical industries.
2.Monoethanolamine is an important corrosion inhibitor used in boiler water treatment, automobile engine coolant, drilling and cutting oil and other types of It acts as a corrosion inhibitor in lubricants. However, monoethanolamine should not be used in combination with nitrite corrosion inhibitors to prevent the formation of nitrosamine carcinogens.
3.Mainly used as absorbent for acidic gases (such as hydrogen sulfide, carbon dioxide, etc.) in petroleum gas, natural gas and other gases . It can also be used as plasticizer, preservative, accelerator, cross-linking agent, cosmetic emulsifier, foaming agent, fabric mothproofing agent, ink additive, printing and dyeing whitening agent, rubber vulcanizing agent, antistatic agent, etc. It is also a raw material for the synthesis of medicines, pesticides, dye intermediates and surfactants.
4.Used as liquid chromatography solvent, eluent and additive. Used as a tail-capping agent when preparing liquid chromatography stationary phases.
5. Mainly used as detergent and solvent detergent, and also used in the preparation of textile printing and dyeing whitening agents and emulsifiers. It is also used as carbon dioxide absorbent, ink additives, petroleum additives, pesticides and pharmaceutical intermediates. It can also be used as a solvent to absorb acidic gases in natural gas, and is used as an emulsifier matrix and penetrating agent in cosmetics.
6.Monoethanolamine can be used as a gas purifier to absorb hydrogen sulfide in natural gas, refinery gas and synthesis gas , carbon dioxide and other acidic gases. Monoethanolamine is an intermediate for the preparation of emulsifiers and is used as antistatic agent, anti-moth agent and detergent for textile processing.

 微信扫一扫打赏
微信扫一扫打赏

