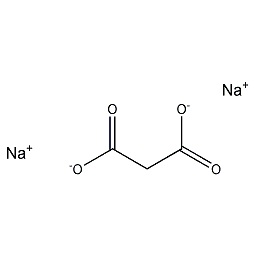
Structural formula
| Business number | 03UL |
|---|---|
| Molecular formula | C3H2Na2O4 |
| Molecular weight | 148.03 |
| label |
sodium malonate, malonate, Malonate sodium salt monohydrate, sodium malate, disodium maleate, Sodiumpropanedioate; Malonic acid disodium salt, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:141-95-7
MDL number:MFCD00002708
EINECS number:205-514-0
RTECS number:OO1750000
BRN number:3917013
PubChem number:24882602
Physical property data
1. Physical property data
1. Appearance: white crystal
2. Solubility: soluble in water, insoluble in alcohol, ether and benzene
Toxicological data
2. Toxicological data:
1. Acute toxicity: rat abdominal LD50: 1100 mg/kg;
Mouse abdominal LD50: 1550 mg/kg
Mouse intravenous LD50: 2100 mg/kg;
Rabbit subcutaneous LDLo: 1584 mg/kg
Rabbit intravenous LD50: 660 mg/kg.
Ecological data
3. Ecological data:
Usually it is not harmful to water. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 80.3
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 72.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 3
Properties and stability
1. Stable under normal temperature and pressure.
2. This product has low toxicity. Rats were intraperitoneally injected with LD501100mg/kg, and rabbits were injected subcutaneously with a minimum lethal dose of 1584mg/kg.
Storage method
Seal and save. Packed in plastic bags and woven bags. Store and transport according to general chemical regulations.
Synthesis method
1. It can be obtained by neutralizing malonic acid and sodium hydroxide solution.
![]()
2. In industrial production, chloroacetic acid is often reacted with sodium cyanide to generate cyanoacetic acid, which is then obtained by the action of liquid alkali Sodium malonate. Dissolve chloroacetic acid in water, neutralize it with saturated sodium carbonate solution below 15°C to pH 7.0, then add sodium cyanide solution at 45°C, react at 105-110°C for 30 minutes, add 45-55 % sodium hydroxide, react at 100-105°C for 1.5h, and the solution is concentrated and dried to obtain sodium malonate.
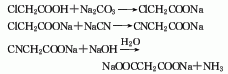
Purpose
1. Raw materials for medicines, dyes and spices. Sodium malonate is esterified with butanol to obtain dibutyl malonate, which is used to produce sulfonamide-6-methoprim; esterified with ethanol to obtain diethyl malonate, which is used to produce the hypnotic drug barbiturate; also Used in the production of synergistic sulfonate.
2. Used in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

