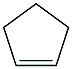
Structural formula
| Business number | 03UY |
|---|---|
| Molecular formula | C5H8 |
| Molecular weight | 68.12 |
| label |
Ester cyclic compounds and their derivatives |
Numbering system
CAS number:142-29-0
MDL number:MFCD00001394
EINECS number:205-532-9
RTECS number:GY5950000
BRN number:635707
PubChem number:24888794
Physical property data
1. Properties: colorless liquid[1]
2. Melting point (℃): -135[2]
3. Boiling point (℃): 44.2[3]
4. Relative density (water=1): 0.774[4]
5. Relative vapor density (air=1): 2.35[5]
6. Saturated vapor pressure (kPa): 42.1 (20℃)[6]
7. Critical temperature (℃): 233[7]
8. Critical pressure (MPa): 4.79[8]
9. Octanol/water partition coefficient: 2.47[9]
10. Flash point ( ℃): -29[10]
11. Ignition temperature (℃): 395[11]
12 . Explosion upper limit (%): 12.1[12]
13. Explosion lower limit (%): 1.5[13]
14. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol, ether, and benzene. [14]
15. Critical density (g·cm-3): 0.274
16. Critical volume (cm 3·mol-1): 248
17. Critical compression factor: 0.284
18. Eccentricity factor: 0.195
19. Lennard-Jones parameter (A): 8.895
20. Lennard-Jones parameter (K): 195.1
21. Solubility parameter (J· cm-3)0.5: 17.156
22. van der Waals area (cm2·mol– 1): 6.050×109
23. van der Waals volume (cm3·mol-1): 46.810
24. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3144.8
25. Gas phase standard claim Heat (enthalpy) (kJ·mol-1): 33.9
26. Gas phase standard entropy (J·mol-1·K -1): 291.38
27. Gas phase standard formation free energy (kJ·mol-1): 111.4
28. Gas phase standard Hot melt (J·mol-1·K-1): 81.28
29. Liquid phase standard combustion heat (enthalpy) (kJ·mol -1): -3115.3
30. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): 4.4
31. Liquid phase standard entropy (J·mol-1·K-1): 2101.25
32. Liquid phase standard generation is free Energy (kJ·mol-1): 180.62
33. Liquid phase standard hot melt (J·mol-1·K– 1):122.38
Toxicological data
1. Acute toxicity:
Oral LD50 in rats: 2140 uL/kg; inhalation LCLo in rats: 16000 ppm/4H; transdermal LD50 in rabbits: 1590 uL/kg.
2. Acute toxicity[15]
LD50: 2.14ml (1656mg)/ kg (rat oral); 1.59ml (1231mg)/kg (rabbit transdermal)
3. Irritation No information available
Ecological data
1. Ecotoxicity[16] LC50: 100mg/L (96h) (fish)
2. Biodegradability No information available
3. Non-biodegradability No information
4. Other harmful effects[17] This substance is harmful to the environment, so special attention should be paid to its effects on the surface Pollution of water, soil, atmosphere and drinking water.
Molecular structure data
1. Molar refractive index: 22.66
2. Molar volume (cm3/mol): 82.0
3. Isotonic specific volume (90.2K ): 191.5
4. Surface tension (dyne/cm): 29.7
5. Polarizability (10-24cm3): 8.98
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 38
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[18] Stable
2. Incompatible substances [19] Strong oxidants, acids, halogenated hydrocarbons, halogens, etc.
3. Conditions to avoid contact[20] Heating and contact with air
4. Polymerization hazard[21] Aggregation
Storage method
Storage Precautions[22] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 29°C. The packaging must be sealed and must not come into contact with air. should be kept away from oxidizer, do not store together. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Preparation method:
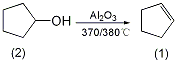
Add anhydrous alumina into a tubular reactor and preheat to 370~380℃. The cyclopentanol is heated to evaporate, and its steam is passed into a preheated reaction tube for thermal cracking. The steam after thermal cracking condenses and separates into a water layer. The obtained oil layer is dried with anhydrous sodium sulfate to obtain cyclopentene①(1), with a yield of 85% to 90%. [24]
2. Preparation method:
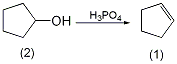
In a reaction flask equipped with a stirrer, fractionation device (the receiving bottle is cooled with an ice bath), and a thermometer, add 50g of 85% phosphoric acid and heat the oil bath to 160 ~170°C, slowly add 250g (2.5mol) of cyclopentanol (2) dropwise, and complete the addition in about 1.5 to 2 hours. After the addition is completed, slowly raise the temperature to 200°C and keep warm for 20 to 30 minutes. The temperature indicated by the thermometer at the top of the fractionating column does not exceed 90°C. Saturate the eluate with salt, separate the organic layer, dry over anhydrous magnesium sulfate, fractionate, collect the fractions at 81-83°C to obtain 165g of cyclopentene (1), with a yield of 80%. Note: 1. Industrially, it can be prepared by partial hydrogenation of cyclopentadiene, which can be separated from the C5 fraction. [25]
Purpose
1. Used in the production of polycyclopentene rubber, cyclopentanediol-1,2- and cyclopentene oxide, the drug ketamine, cyclopentylphenol (disinfectant), cyclic aldehydes, cyclopentanediol, etc. .
2. Used as comonomer, also used in organic synthesis, as cross-linking agent for resin.
3. Used as solvents and laboratory reagents. [23]

 微信扫一扫打赏
微信扫一扫打赏

