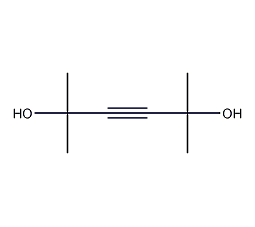
Structural formula
| Business number | 03UZ |
|---|---|
| Molecular formula | C8H14O2 |
| Molecular weight | 142.20 |
| label |
2,5-dimethyl-2,5-dihydroxy-3-hexyne, 2,5-dimethyl-2,5-hexynediol, 2,5-Dimethyl-3-hexyne-5-diol |
Numbering system
CAS number:142-30-3
MDL number:MFCD00004468
EINECS number:205-533-4
RTECS number:MR0176000
BRN number:969835
PubChem ID:None
Physical property data
1. Physical property data:
1. Appearance: white liquid or brown crystal
2. Density (g/mL, 20℃): 0.949
3. Melting point (ºC): 90-94ºC
4. Boiling point (ºC, normal pressure): 205-206ºC
5. Flash point (ºC): 102
6. Solubility: Solubility in water at 20°C is 27.0%, soluble in acetone, ethylene glycol, and methyl ethyl ketone.
7. The standard heat of combustion (enthalpy) of the crystal phase (kJ·mol-1): -4763.2
8. The standard claim heat of the crystal phase ( Enthalpy) (kJ·mol-1): -385.7
Toxicological data
2. Toxicological data:
1. Acute toxicity: mouse abdominal LD: >500 mg/kg
Ecological data
3. Ecological data:
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 40.11
2. Molar volume (cm3/mol): 139.0
3. Isotonic specific volume (90.2K): 352.2
4. Surface tension (dyne/cm): 41.2
5. Polarizability (10-24cm3): 15.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.1
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 160
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure.
Incompatible materials: strong oxidizing agents
Storage method
Keep sealed.
Synthesis method
Obtained from the reaction of acetone and acetylene. ShouldThe condensation reaction is carried out in the solvent benzene, in the presence of potassium hydroxide at 35-40°C, and then acidified with hydrochloric acid to obtain the finished product.
Purpose
1. Used as surfactant and polyol component of polyester in polyurethane production. It is also an intermediate for herbicides, adhesives, metal surface treatment additives, lubricants for wires, organic peroxides and defoaming agents.
2. Semi-bright nickel intermediate.
3. Used as raw materials for brighteners and spices, herbicides and pesticides in the electroplating industry.

 微信扫一扫打赏
微信扫一扫打赏

