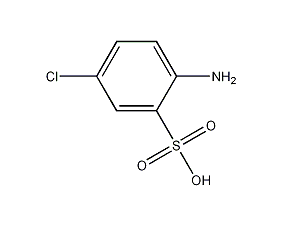
Structural formula
| Business number | 03P7 |
|---|---|
| Molecular formula | C6H6ClNO3S |
| Molecular weight | 207.63 |
| label |
5-Chloroorthanilic acid, 4-Chloroaniline-2-sulfonic acid, aromatic compounds |
Numbering system
CAS number:133-74-4
MDL number:None
EINECS number:205-119-3
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
None yet
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1、 Molar refractive index:45.54
2, Molar volume(m3/mol):126.4
3, Isotonic specific volume(90.2K):365.3
4, Surface tension(3.0 dyne/cm SPAN>): 69.7
5, Polarizability( 0.5 10-24cm3): 18.05
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 88.8
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 248
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
Obtained from the sulfonation of p-chloroaniline: slowly drop 100% sulfuric acid into p-chloroaniline and the solvent diphenyl sulfone, and react violently to generate 4-chloroaniline-2-sulfonic acid and water. When the water was evaporated, the reaction mixture turned into bright purple crystals. Continue heating under reduced pressure for 7 hours, cool the melt, dissolve in heated water, filter out the diphenyl sulfone in the solution, and cool to obtain white needle crystals. Filter, wash and dry to obtain the finished product. The yield is 94%.
Purpose
Used as an intermediate in organic synthesis.
ng=EN-US style=”FONT-SIZE: 9pt; FONT-FAMILY: Arial; mso-font-kerning: 0pt”>18.05
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 88.8
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 248
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
Obtained from the sulfonation of p-chloroaniline: slowly drop 100% sulfuric acid into p-chloroaniline and the solvent diphenyl sulfone, and react violently to generate 4-chloroaniline-2-sulfonic acid and water. When the water was evaporated, the reaction mixture turned into bright purple crystals. Continue heating under reduced pressure for 7 hours, cool the melt, dissolve in heated water, filter out the diphenyl sulfone in the solution, and cool to obtain white needle crystals. Filter, wash and dry to obtain the finished product. The yield is 94%.
Purpose
Used as an intermediate in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

