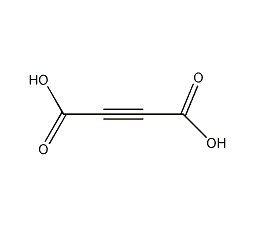
Structural formula
| Business number | 03V1 |
|---|---|
| Molecular formula | C4H2O4 |
| Molecular weight | 114.06 |
| label |
2-Butynedioic acid, Acetylene-1,2-dicarboxylic acid, 2-Butynedioic acid |
Numbering system
CAS number:142-45-0
MDL number:MFCD00004362
EINECS number:205-536-0
RTECS number:None
BRN number:878357
PubChem number:24886837
Physical property data
None yet
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 21.71
2. Molar volume (cm3/mol): 67.1
3. Isotonic specific volume (90.2K ): 213.0
4. Surface tension (dyne/cm): 101.4
5. Polarizability (10-24cm3): 8.60
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.1
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 74.6
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 161
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Storage method
None
Synthesis method
1. Using α,β-dibromosuccinic acid as raw material, remove HBr in an alkaline solution to obtain sodium butyndioic acid. Preparation process of sodium butyndioic acid: Prepare sodium hydroxide solution, cool it, and add α,β-dibromosuccinic acid one by one. After the addition is completed, raise the temperature to 40°C and keep warm for 3 hours. Cool the crystallization to below 10°C, filter, and dry the crystal to obtain sodium butynioate. The yield is 75-87%.
2. Preparation method:
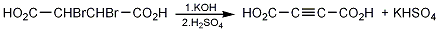
p>
Into a 2L reaction bottle equipped with a stirrer and reflux condenser, add 122g (2.2mol) of potassium hydroxide and 700mL of 95% methanol, and stir to dissolve. Slowly add 100g (0.36mol) of a,β-dibromosuccinic acid (2)①, and heat to reflux for 1.5h. After cooling and suction filtration, the filter cake is fully washed with methanol and dried to obtain 144-150g of mixed salt. Dissolve the mixed salt in 270 mL of water, add dilute acid composed of 30 mL of water and 8 mL of concentrated sulfuric acid, and the monopotassium salt of butynodiic acid precipitates, and leave it overnight. Filter with suction, dissolve the acid salt in dilute acid made of 60 mL concentrated sulfuric acid and 240 mL water, and extract with ether (100 mL × 5). Combine the ether extracts and evaporate the ether to obtain butynedioic acid hydrate. Vacuum dry in a desiccator filled with concentrated sulfuric acid to obtain 30-36g of butynedioic acid (1), yield 73%-88%, mp 175-177.�. Note: ① The reaction is exothermic and cannot be added at once. [1]
Purpose
Pharmaceutical intermediates, used in the production of antidote sodium dimercaptosuccinate.

 微信扫一扫打赏
微信扫一扫打赏

