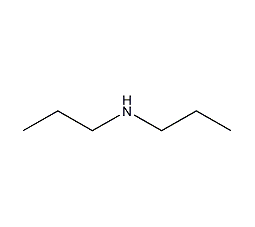
Structural formula
| Business number | 03VG |
|---|---|
| Molecular formula | C6H15N |
| Molecular weight | 101 |
| label |
di-n-propylamine, N-dipropylamine, N-Propyl-1-propanamine, Di-n-propylamine, N-Dipropylamine, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:142-84-7
MDL number:MFCD00009362
EINECS number:205-565-9
RTECS number:JL9200000
BRN number:505974
PubChem ID:None
Physical property data
1. Properties: colorless transparent liquid with ammonia odor. [1]
2. Melting point (℃): -63[2]
3. Boiling point (℃): 109.3[3]
4. Relative density (water = 1): 0.738[4]
5. Relative vapor Density (air=1): 3.49[5]
6. Saturated vapor pressure (kPa): 2.68 (25℃)[6]
7. Heat of combustion (kJ/mol): -4411.9[7]
8. Critical temperature (℃): 277[8]
9. Critical pressure (MPa): 3.14[9]
10. Octanol/water partition coefficient: 1.67 [10]
11. Flash point (℃): 17[11]
12. Ignition temperature (℃): 299 [12]
13. Solubility: soluble in water, miscible in ethanol, ether, benzene and acetone. [13]
14. Refractive index (20ºC): 1.4045
15. Viscosity (mPa·s, 20.1ºC): 0.5335
16. Flash point (ºC): 299
17. Heat of evaporation (KJ/mol, 25ºC): 40.86
18. Heat of evaporation (KJ/mol, b.p.): 34.90
19. Specific heat capacity (KJ/(kg·K), constant pressure): 2.5
20.pKa (25ºC, water): 11.00
Toxicological data
1. Acute toxicity:
Rat oral LD50: 460 mg/kg; rat intraperitoneal LDLo: 75 mg/kg;
Rat LD50 has not been reported: 280 mg/kg; not reported LD50 in mice: 320 mg/kg;
Rabbit transdermal LDLo: 1250 uL/kg; Rabbit subcutaneous LD50: 1250 mL/kg.
2. Inhalation toxicity: rat LC50: 4400 mg/m3/4H; mouse LC50: 3070 mg/m3/2H.
3. Acute toxicity[14]
LD50: 300mg/kg (rat Oral); 925mg/kg (rabbit transdermal)
LC50: 4400mg/m3 (rat inhalation, 4h)
4 .Irritation[15] Rabbit transdermal: 100μg (24h), causes irritation (open irritation test).
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability[16] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 4h (theoretical).
4. Other harmful effects[17] This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 33.44
2. Molar volume (cm3/mol): 136.3
3. Isotonic specific volume (90.2K ): 298.8
4. Surface tension (dyne/cm): 23.0
5. Polarizability (10-24cm3): 13.25
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 12
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 23.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It has the chemical properties of secondary amines. The aqueous solution is alkaline, forming C6H15N·0.5H2O and C6H15N·H2O.
2. This product is highly toxic and highly irritating to skin and tissues. Rat oral LD501.87mg/kg. The maximum allowable concentration in the operating location is 12mg/m3. Protective equipment should be worn during operation. The equipment should be sealed and prevented from running, popping, dripping, and leaking. Pay attention to safety.
3. Stability[18] Stable
4. Incompatible substances[19] Strong oxidants, acids
5. Polymerization hazard[20] No polymerization
Storage method
1. Storage precautions[21] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants and acids, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. This product is flammable, with an explosion limit of 2.0% to 10.4% in air. It can be shipped in barrels or tankers, and the packaging container must be Sealed, stored in a cool and ventilated place, strictly prohibited from fireworks, and stored isolated from oxidants. Store and transport according to regulations for flammable and toxic substances.
Synthesis method
1. 1. n-Propanol amination method uses propanol as raw material and obtains it through catalytic dehydrogenation, ammoniation, dehydration and hydrogenation (see “tripropylamine”). According to relevant data reports, the above reaction uses nickel – Copper-pumice is used as a catalyst, which is beneficial to the generation of dipropylamine; using nickel-copper-activated alumina as a catalyst is beneficial to the generation of tripropylamine. 2. The acrylonitrile hydrogenation method uses acrylonitrile as raw material, copper-nickel compounds as catalysts, and performs catalytic hydrogenation at a temperature of 40-250°C and a pressure of 0-4.9MPa to obtain dipropylamine. In addition, when hydrogenating propionitrile or acrylonitrile using a rhodium catalyst with carbon as a carrier and using excess hydrogen to continuously remove ammonia, the selectivity to generate dipropylamine is over 85%, n-propylamine is rarely generated, and tripropylamine is not generated at all. . A high proportion of dipropylamine is also obtained by reductive amination of propionaldehyde under the action of rhodium catalyst.

2. The preparation method is as follows Propanol is used as raw material and is obtained through catalytic dehydrogenation, ammoniation, dehydration and hydrogenation. The reaction catalyst is Ni-Cu-Al2O3, the pressure is (39±1)kPa, the reactor temperature is (190±10)℃, the space velocity of propanol is 0.05~0.15h-1, and the raw material ratio is propanol:ammonia ∶Hydrogen = 4:2:4, dipropylamine and tripropylamine are obtained at the same time, and dipropylamine can be obtained by fractionation.
![]()
Purpose
1. Used as raw materials for organic synthesis. Used for the preparation of pesticides, medicines (dipropylglutamide, etc.), boiler antiseptics, engine coolants, lubricating oils, metal cutting oils, carbon removers, anti-corrosion lubricants and emulsifiers, and solvents, etc. Among them, pesticides are the most important use. The main pesticides produced by dipropylamine are: trifluralin, dipropylamine, fenclofen, fenclofen, sulfonalin, isopropylalin, methylalin, fenclofen, and fenclofen.
2. Used as organic synthesis intermediates and solvents. [22]

 微信扫一扫打赏
微信扫一扫打赏

