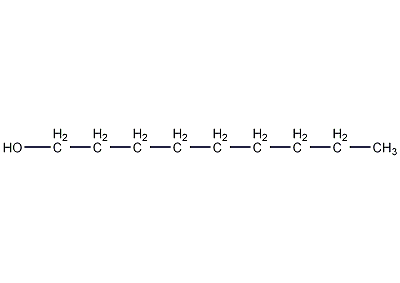
Structural formula
| Business number | 03VR |
|---|---|
| Molecular formula | C9H20O |
| Molecular weight | 144.25 |
| label |
n-nonanol, Nine carbon alcohol, Geranium alcohol, Nonan-1-ol, Nonoyl alcohol, n-Nonanol, Solvent for nitrocellulose spray paint and enamel paint, D, defoamer, surfactant, alcohol solvent |
Numbering system
CAS number:143-08-8
MDL number:MFCD00002990
EINECS number:205-583-7
RTECS number:RB1575000
BRN number:969213
PubChem number:24886672
Physical property data
1. Properties: colorless to light yellow viscous liquid with slight rose scent.
2. Boiling point (ºC, 101.3kPa): 213.1
3. Melting point (ºC): -5
4. Relative density (g/mL, 20/4ºC): 0.8280
5. Refractive index (n20ºC): 1.4338
6. Viscosity (mPa·s,0ºC): 56.0
7. Viscosity (mPa·s, 20ºC): 14.3
8. Flash point (ºC, closed): 99
9. Heat of evaporation (KJ/kg): 561.0
10. Heat of combustion (KJ/mol): 5946.1
11. Specific heat capacity (KJ/(kg·K), 13ºC, constant pressure): 2.34
12. Steam Pressure (kPa, 20ºC): 0.40
13. Thermal conductivity (W/(m·K), 20ºC): 16.75
14. Freezing point (ºC): -5
15. Solubility (%, 20ºC, water): 0.06
16. Volume expansion coefficient (K-1): 0.00091
17. Solubility: Slightly soluble in water, soluble in ethanol and ether, miscible in alcohol, ether and chloroform.
18. Relative density (25℃, 4℃): 0.8247
19. Refractive index at room temperature (n25): 1.4319
20. Critical temperature (ºC): 366.85
21. Critical pressure (MPa): 2.528
22. Critical density (g·cm-3): 0.252
23. Critical volume (cm3·mol-1): 572
24. Critical compression Factor: 0.260
25. Eccentricity factor: 0.594
26. Solubility parameter (J·cm-3)0.5 :20.582
27. van der Waals area (cm2·mol-1): 1.438×1010
28. van der Waals bodyProduct (cm3·mol-1): 103.550
29. Gas phase standard combustion heat (enthalpy) (kJ·mol-1 ): 6023.16
30. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -376.73
31. Liquid Phase standard combustion heat (enthalpy) (kJ·mol-1): -5946.30
32. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1 ): -453.59
33. Liquid phase standard hot melt (J·mol-1·K-1): 380.6
Toxicological data
1. Acute toxicity: Rat oral LD50: 3560 mg/kg;
Rat intraperitoneal LD50: 800 mg/kg;
Mouse oral LD50: 6400 mg/kg;
Mouse intraperitoneal LD50: 800 mg/kg;
Rabbit transdermal LD50: 5660 uL/kg.
2. Inhalation toxicity: mouse LC50: 5500 mg/m3/2H. Animal experiments have found that it can cause central nervous system and liver disorders.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 45.27
2. Molar volume (cm3/mol): 174.6
3. Isotonic specific volume (90.2K ): 406.9
4. Surface tension (dyne/cm): 29.5
5. Polarizability (10-24cm3): 17.95
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 7
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 52.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure, non-corrosive to metals. Typical reactivity of higher primary alcohols.
2. Incompatible materials: strong oxidizing agents.
3. Found in flue-cured tobacco leaves.
4. Naturally found in grapefruit oil, sweet orange oil, rose oil, apples, cooked beef, and cheese.
Storage method
Store sealed in a dry and cool place. Can be stored in iron, mild steel, copper or aluminum containers.
Synthesis method
1. There are many production methods in industry. Propylene is polymerized in the presence of phosphoric acid or boron fluoride to obtain nonene, and nonene can be hydrated to obtain nonanol. The isobutene in the butane-butene fraction is dimerized to obtain diisobutylene and mixed octene, and then carbonylated to obtain nonanol and mixed nonanol. Nonanol is obtained by reducing ethyl nonanoate with metallic sodium in an ethanol solution.
2. Nonanol can be obtained by the following reaction between heptane bromide and ethylene oxide.
3. Obtained from the reduction of ethyl nonanoate and metallic sodium in ethanol solution.
4. Tobacco: FC, 40.
5. Preparation method:
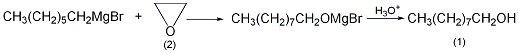
First use 24.5g (1mol) magnesium chips, 179g (1.0mol) 1-bromoheptane and 300mL dibutyl ether ① to prepare Grignard reagent according to the conventional method. Cool to 0°C, add filtered ethylene oxide (2) under vigorous stirring, keep the temperature of the reaction system at about 0°C, and react for 1 hour after the passage is completed. Then the temperature was raised to 40°C and reacted for 1 hour. Then heat the reaction in a water bath for 2 hours. Cool, pour the reactant into ice water, and acidify with dilute sulfuric acid to dissolve the generated magnesium hydroxide. Treat ammonium with the above-mentioned 1-hexanol treatment method, collect the fraction at 95~100℃/1.6kPa, and obtain 95g of 1-nonanol (1), with a yield of 69%. Note: ① The purification method of dibutyl ether is as follows: wash with sodium hydroxide solution and water in sequence, dry with anhydrous calcium chloride, and then fractionate to collect the fractions between 140 and 142°C. [1]
Purpose
1. Used as a solvent, wetting agent, defoaming agent, etc. for nitrocellulose spray paint and enamel paint. It is also used as a raw material for the manufacture of surfactants and spices.
2. Used as a solvent to produce plasticizers, surfactants, stabilizers, and defoaming agents. This product has a slightly pleasant aroma of rose and orange. The natural product exists in free or esterified state in essential oils such as sweet orange, bitter orange, grapefruit and oakmoss. Our country’s GB2760-86 stipulates that the edible flavors are allowed to be used, and they are mainly used for this type of cream, peach, orange, lemon, white lemon and pineapple flavors. The dosage is very small.
3. Commonly used in baked goods, cheese, and frozen dairy products.

 微信扫一扫打赏
微信扫一扫打赏

