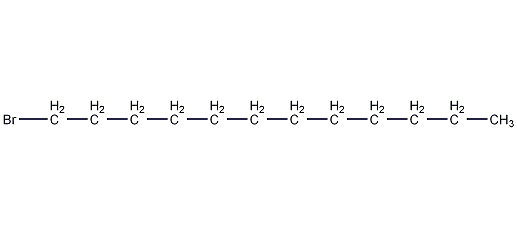
Structural formula
| Business number | 03VU |
|---|---|
| Molecular formula | C12H25Br |
| Molecular weight | 249.23 |
| label |
1-Bromo-n-dodecane, 1-bromododecane, 1-bromo-n-dodecane, 1-Lauryl bromide, Dodecyl bromide, Bromododecane, Lauryl bromide, n-Dodecyl bromide, Aliphatic halogenated derivatives |
Numbering system
CAS number:143-15-7
MDL number:MFCD00000225
EINECS number:205-587-9
RTECS number:None
BRN number:506159
PubChem number:24850636
Physical property data
1. Properties: colorless to light yellow liquid. Has a coconut smell.
2. Relative density (g/mL, 20/4℃): 1.0399
3. Melting point (ºC): -5.5
4. Boiling point ( ºC, normal pressure): 276
5. Boiling point (ºC, 1.33kPa): 139
6. Refractive index (n20ºC): 1.4583
7. Flash point (ºC): 110
8. Solubility: Miscible with ethanol, ether and acetone, insoluble in water.
9. Relative density (25℃, 4℃): 1.0355
10. Refractive index at room temperature (n25): 1.4564
Toxicological data
It is toxic. Long-term inhalation can cause neurological disorders and has anesthetic effects.
Ecological data
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 65.37
2. Molar volume (cm3/mol): 239.7
3. Isotonic specific volume (90.2K ): 561.7
4. Surface tension (dyne/cm): 30.1
5. Polarizability (10-24cm3): 25.91
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 10
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 81.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure. When exposed to open flames or exposed to high heat, it will burn and decompose to release toxic gases.
Incompatible materials: strong oxidizing agents
Storage method
Store sealed and away from light.
Synthesis method
1. Obtained from the reaction between lauryl alcohol (n-dodecanol) and hydrobromic acid: Add lauryl alcohol to the enamel glass reaction pot, stir and cool, and slowly add sulfuric acid. Stir for 1 hour, then add hydrobromic acid. The temperature was gradually increased to 90~95°C, and the reaction was stirred for 8 hours. After the reaction is completed, the temperature is lowered to 30°C and left to stand for 8 hours. Separate the acid layer, adjust the pH of the oil layer to 8 with dilute alkali solution, remove the alkali solution, and wash with 50% ethanol. Distill under reduced pressure and collect the 140~200℃ (3.33kPa) fraction to obtain the product. The yield is over 90%.
2. Preparation method:
![]()
In a reaction bottle equipped with a stirrer, thermometer and ventilation tube, add 186g (1.0mol) of n-dodecyl alcohol (2), heat to 100°C with stirring, and pass in dry hydrogen bromide gas , keep the reaction temperature between 100 and 120°C until the solution no longer absorbs hydrogen bromide. After cooling, add 70 mL of water, shake thoroughly, and separate the water layer. Add 1/3 volume of concentrated sulfuric acid to the organic layer, shake it thoroughly, separate the acid layer, wash with water, 10% sodium carbonate, and 50% methanol in sequence ①, dry over anhydrous calcium chloride, and reduce Pressure distillation, collect the fractions at 130~135/0.8kPa, and obtain 220g of 1-bromododecane (1)②, with a yield of 88%. Note: ① Washing with 50% methanol can prevent emulsification and the loss of product is very small. ② It can also be prepared with 50% hydrobromic acid in the presence of sulfuric acid, with a yield of about 90%. [1]
Purpose
1. Mainly used in organic synthesis, and in medicine for the synthesis of disinfectants such as chlormethionine and domiphene.
2. Used as a solvent, it can also be used to synthesize the flame retardant tris(dodecylmercaptan) antimony, the antioxidant dodecylmercaptan, and the surfactant dodecyldimethyl Benzyl ammonium chloride (benzyl ammonium chloride), benzalkonium bromide, and other flame retardants and surfactants.

 微信扫一扫打赏
微信扫一扫打赏

