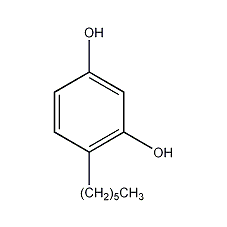
Structural formula
| Business number | 03R1 |
|---|---|
| Molecular formula | C12H18O2 |
| Molecular weight | 194.27 |
| label |
hexyl resin powder, 4-Hexyl-1,3-dihydroxybenzene, 1,3-dihydroxy-4-hexylbenzene, preservative, Antioxidants, food additives, Pigment stabilizer |
Numbering system
CAS number:136-77-6
MDL number:MFCD00002284
EINECS number:205-257-4
RTECS number:VH1575000
BRN number:2048312
PubChem number:24852569
Physical property data
1. Properties: off-white or yellow-white needle-like crystals (solid at room temperature). It has a faint fatty odor and a strong astringent taste, and causes numbness on the tongue. It has been oxidized in the air or exposed to light and turned light brown or pink.
2. Melting point (℃): 65~67
3. Boiling point (℃): 333
4. Solubility: slightly soluble in water. Easily soluble in ethanol, methanol, glycerol, ether, chloroform, benzene and vegetable oil.
Toxicological data
1. Acute toxicity data:
Oral LD50 in rats: 550mg/kg;
Oral LD50 in mice: 1040mg/kg;
Mouse abdominal LD50: 335mg/kg;
Mouse subcutaneous LDLo: 750mg/kg;
Mouse route unknown LDLo: 100mg/kg;
Guinea pig Oral LDLo: 440mg/kg;
2. Other multiple dose data
Oral TDLo in rats: 16250mg/kg/13W-I;
Small Oral TDLo in mice: 32500mg/kg/13W-I;
3. Oncogenicity data: Oral TDLo in mice: 64mg/kg/2Y-C;
4. Reproduction Data: Subcutaneous exposure to TDLo 1 day before mating in female rats: 5mg/kg;
5. Mutagenicity data: Bacteria-E. coli DNA repair system: 3mg/disc; Mouse lymphocytes: 5ug/L .
6. Skin/eye irritation data: Rabbit eye contact: 2mg
Ecological data
None
Molecular structure data
1. Molar refractive index: 58.09
2. Molar volume (cm3/mol): 185.1
3. Isotonic specific volume (90.2K ): 473.0
4. Surface tension (3.0 dyne/cm): 42.6
5. Polarizability (0.5 10-24cm 3): 23.03
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: 9
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 147
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and StabilitySex
It has strong astringency and can make it lose consciousness when placed on the tongue.
Storage method
None
Synthesis method
1. Obtained from the reduction of caproylresorcinol. Add zinc amalgam to hexanoyl resorcinol, then add industrial hydrochloric acid, stir and raise the temperature to 75-80°C, naturally raise the temperature to 104-110°C, keep the reaction for 1.5-2 hours, cool to 80°C, and check the reaction end point. After the reaction is completed, the temperature is lowered to below 40°C, the reduced product is separated, washed with water, and the water is evaporated under reduced pressure. The 145-152°C (0.133-0.267kPa) fraction is collected to obtain crude 4-hexylresorcinol. The yield is 90%. The crude product is recrystallized with petroleum ether to obtain the finished product.
2. Put 1/2 of the caproic acid into the dissolving pot, add anhydrous zinc chloride, and heat and stir until the mixture dissolves at about 120°C; put the other half of the caproic acid into the condensation pot. And add resorcinol, stir and dissolve; add the above zinc chloride/caproic acid solution dropwise at about 120°C, reduce the pressure to 93kPa, keep the reaction for 3 hours, and at the same time evaporate the water generated by the reaction; after the reaction is completed, lower the temperature to 80 ℃, add water and wash 5 times, move it to a distillation pot, dehydrate under constant pressure, dehydrate under reduced pressure, and recover unreacted caproic acid to obtain the condensation product hexanoyl resorcinol. Add zinc amalgam to hexanoyl resorcinol, then add industrial hydrochloric acid, stir and raise the temperature to 75-80°C, naturally raise the temperature to 104-110°C, and keep the reaction for 1.5-2.0 hours. Cool the temperature to 80°C and check the end point of the reaction; after the reaction is completed, cool the temperature to below 40°C and separate out the reduced substance. After washing with water, evaporate the water under reduced pressure and collect the 145-152°C (133-266Pa) fraction to obtain 4-hexylisophenylene. Crude phenol, yield 90%; the crude product is recrystallized with petroleum ether to obtain the finished product.
3. It is made by condensation of hexanoic acid and resorcinol, and then refined by reduction and distillation of zinc amalgam.
Purpose
This product is used as an antioxidant, mainly used in the processing of shrimp and crab aquatic products. Its purpose is to prevent oxidative browning or blackening of the product due to the catalysis of polyphenol oxidase during storage. phenomenon occurs. As well as insect repellents and food additives.

 微信扫一扫打赏
微信扫一扫打赏

