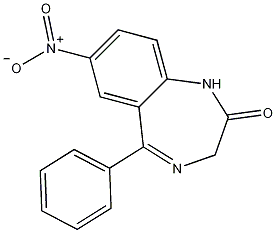
Structural formula
| Business number | 03WX |
|---|---|
| Molecular formula | C15H11N3O3 |
| Molecular weight | 281.27 |
| label |
1,3-Dihydro-7-nitro-5-phenyl-2H-1,4-benzodiazepin-2, 5-phenyl-7-nitro-1,3-dihydro-2H-1,4-benzodiazepin-2-one, 1,3-Dihydro-7-nitro-5-phenyl-2h-4-benzodiazepin-2-one |
Numbering system
CAS number:146-22-5
MDL number:MFCD00058577
EINECS number:205-665-2
RTECS number:DF2450000
BRN number:None
PubChem number:24897666
Physical property data
1. Physical property data:
1. Characteristics: Light yellow flake crystal
2. Solubility:Soluble in ethanol, acetone, chloroform, ethyl acetate, methylene chloride, insoluble in water, ether, benzene and hexane
3. Melting point (ºC): 226-229ºC (decomposition)
Toxicological data
2. Toxicological data:
1, acute toxicity: rat oral LD50: 825 mg/kg;
Rat abdominal cavity LD50: 733 mg/kg;
Mouse oral LD50: 550 gm/kg;
Mouse abdominal cavity LD50: 275 mg/kg;
Mouse subcutaneous LD50: >400 mg/kg;
Mouse intravenous LD50: 130 mg/kg;
Rabbit oral LD50: 520 mg/kg.
Ecological data
3. Ecological data:
Other harmful Effect: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data: 1. Molar refractive index:76.64 2. Molar volume (m3/mol):200.6 3. Isotonic specific volume (90.2K):559.7 4. Surface tension (dyne/cm):60.6 5. Polarizability(10-24cm3):30.38
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 10
6. Topological molecule polar surface area 87.3
7. Number of heavy atoms: 21
8. Surface charge: 0
9. Complexity: 452
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure.
Storage method
Seal in a dry and cool place: Arial”>2. Molar volume ( m3/mol): 200.6
3. Isotonic specific volume (90.2K):559.7
4. Surface tension (dyne/cm):60.6
5. Polarizability(10-24cm3):30.38
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 10
6. Topological molecule polar surface area 87.3
7. Number of heavy atoms: 21
8. Surface charge: 0
9. Complexity: 452
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure.
Storage method
Seal and store in a dry and cool place.
Synthesis method
2-Amino-5-Nitrobenzophenone (see 02420) dissolved in dry benzene, add chloroacetyl chloride dropwise with stirring, raise the temperature and reflux 4h to generate 2-Chloroacetamide-5-Nitrobenzophenone. The base, hexamethylenetetramine and ethanol are heated to reflux 20h, and then the ethanol is recovered by distillation, filtered to obtain the crude product, and then recrystallized to obtain the finished product of nitrazepam.
Purpose
This product is a benzodiazepine drug with hypnotic, muscle relaxant, anticonvulsant and anti-inflammatory properties. Anxiety effect. Take the medicine 15-30min and fall asleep, which lasts 6-8h, which is close to natural sleep.
�Save.
Synthesis method
2-Amino-5-Nitrobenzophenone (see 02420) dissolved in dry benzene, add chloroacetyl chloride dropwise with stirring, raise the temperature and reflux 4h to generate 2-Chloroacetamide-5-Nitrobenzophenone. The base, hexamethylenetetramine and ethanol are heated to reflux 20h, and then the ethanol is recovered by distillation, filtered to obtain the crude product, and then recrystallized to obtain the finished product of nitrazepam.
Purpose
This product is a benzodiazepine drug with hypnotic, muscle relaxant, anticonvulsant and anti-inflammatory properties. Anxiety effect. Take the medicine 15-30min and fall asleep, which lasts 6-8h, which is close to natural sleep.

 微信扫一扫打赏
微信扫一扫打赏

