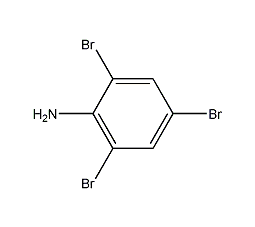
Structural formula
| Business number | 03X9 |
|---|---|
| Molecular formula | C6H4Br3N |
| Molecular weight | 329.81 |
| label |
1,4,6-Tribromobenzenamine |
Numbering system
CAS number:147-82-0
MDL number:MFCD00007634
EINECS number:205-700-1
RTECS number:BZ0175000
BRN number:2209258
PubChem number:24850033
Physical property data
1. Physical property data:
1. Properties: Witch-colored to brown-yellow needle-like crystals.
2. Boiling point (℃): 300℃.
3. Melting point (℃): 122℃.
4. Relative density (g/mL, water=1): 2.35.
5. Flash point (ºC): 300 ºC
6. Solubility: Insoluble in water, soluble in cold water, ethanol, chloroform, ether, etc.
Toxicological data
2. Toxicological data:
1. Acute toxicity: mouse abdominal LD50: 500 mg/kg
Ecological data
3. Ecological data:
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 53.55
2. Molar volume (cm3/mol): 140.2
3. Isotonic specific volume (90.2K): 384.5
4. Surface tension (dyne/cm): 56.5
5. Polarizability (10-24cm3): 21.23
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.3
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 108
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure.
Incompatible materials: acids, acid chlorides, acid anhydrides, chloroform, strong oxidants.
Storage method
Store sealed in a dry and cool place.
Synthesis method
Add 10g (0.11mol) aniline, 100mL water and 10mL concentrated hydrochloric acid into a 1L flask, shake to dissolve the aniline, and dilute with 400mL water. A gas introduction tube is installed on the flask stopper, close to the bottom of the bottle. The top of the tube is connected to the branch tube of the 100 mL branch flask. The branch flask contains 60 g of bromine (19 mL, 0.38 mol) and is immersed in a water bath maintained at a temperature of 30~40°C. An additional air outlet pipe is installed on the plug of the reaction flask, which is connected to a hydrogen bromide absorption device with an air pump. Evacuate gently and slowly pass the bromine vapor into the aniline hydrochloride solution until the solution in the reaction flask shows bromine color (it takes about 2 to 3 hours). After the reaction is completed, filter out the tribromoaniline crystals.Wash the adsorbed hydrobromic acid with water, filter and press dry as much as possible. Recrystallized once with ethanol, yield 22g, yield 63%, melting point 120°C.
Purpose
1. Used as analytical reagents and dye synthesis.
2. Used as pharmaceutical intermediates, chemical flame retardants, etc.
3. Organic synthesis intermediates.

 微信扫一扫打赏
微信扫一扫打赏

