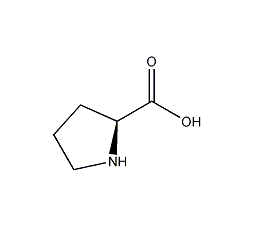
Structural formula
| Business number | 03XA |
|---|---|
| Molecular formula | C5H9NO2 |
| Molecular weight | 115.13 |
| label |
L-pyrrolidine-2-carboxylic acid, Hydrogenated pyrrocarboxylic acid, 2-pyrrolidinecarboxylic acid, (S)-(-)-proline, (S)-(-)-Proline, 2-Pyrrolidinecarboxylic acid, amino acid drugs, intermediates, Biochemical reagents |
Numbering system
CAS number:147-85-3
MDL number:MFCD00064318
EINECS number:205-702-2
RTECS number:TW3584000
BRN number:80810
PubChem number:24898097
Physical property data
1. Properties: colorless crystals
2. Density (g/mL, 20℃): 1.186
3. Melting point (ºC): 221
4. Specific rotation (º): -85º
5. Solubility: easily soluble in water and ethanol, insoluble in butanol and ether.
Toxicological data
None
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 27.90
2. Molar volume (cm3/mol): 96.9
3. Isotonic specific volume (90.2K): 249.0
4. Surface tension (dyne/cm): 43.4
5. Polarizability (10-24cm3): 11.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 49.3
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 103
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure.
2. Incompatible materials: strong oxidizing agents.
3. Exist in tobacco leaves and smoke.
Storage method
Freeze-drying and preservation.
Synthesis method
1. Hydrolyzates of proteins such as gelatin and casein are treated with ion exchange resin, and then the neutral amino acid part is treated with picric acid or Reineckeates salt to precipitate only L-proline. Finally, it is obtained by recrystallization with absolute ethanol and isopropyl alcohol.
Corynebacteriumacetoacidophilum XQ-3 (selected by the Central Research Institute of Wuxi Light Industry UniversityIngredients and food additives.
5. Proline itself is chiral, so it is often used as a chiral leading reagent in the total synthesis of some natural products; at the same time, as an optically active auxiliary agent, proline can be used in various It plays an important role in symmetric total synthesis[1].
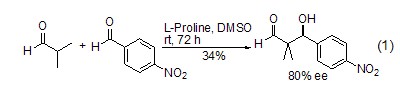
Asymmetric Catalysis When proline is used as a chiral catalyst, carbonyl compounds can undergo an asymmetric Michael reaction, and the product is β-hydroxyaldehyde (or ketone) (Formula 1)[2~4].
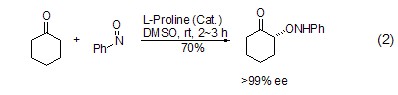
Catalyzed by L-proline Under the conditions, nitroso compounds can undergo α-amine oxidation reaction with carbonyl compounds (Formula 2) [5,6].
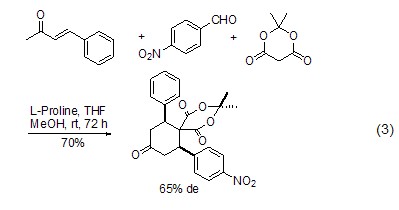
L-proline can also be used Catalytic asymmetric Diels-Alder reaction (Formula 3)[7].
In addition, as an asymmetric synthesis As a chiral catalyst, L-proline can also catalyze Aldol[8~10], α-amination[11,12], α-alkylation[13] and other reactions.
Catalyze the synthesis of carbon-carbon and carbon-nitrogen In addition to being a chiral catalyst to catalyze asymmetric synthesis, proline can also be used as a copper ligand to catalyze carbon-carbon synthesis. The formation of bond (Formula 4)[14] and carbon-nitrogen bond (Formula 5)[15].
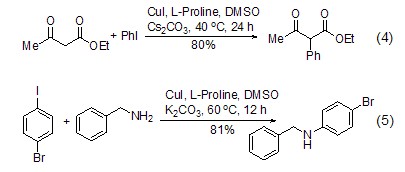
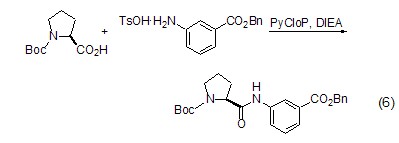
Formation of peptide bonds L-proline is similar to other amino acids and can also form peptide bonds (Formula 6)[16], and because the amino group and carboxyl group in the L-proline molecule are in a cis structure , conducive to the formation of cyclic peptides.

 微信扫一扫打赏
微信扫一扫打赏

