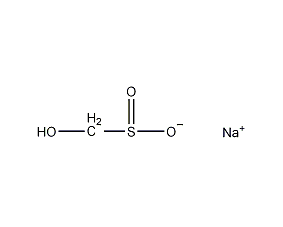
Structural formula
| Business number | 03XS |
|---|---|
| Molecular formula | NaH7SCO5 |
| Molecular weight | 154.12 |
| label |
sodium formaldehyde sulfoxylate, Hydroxymethanesulfinic acid monosodium salt, carved white block, Sodium formaldehyde sulfoxylate hydrate, dye discharge agent, reducing agent |
Numbering system
CAS number:149-44-0
MDL number:MFCD00150599
EINECS number:205-739-4
RTECS number:PB0380000
BRN number:3630814
PubChem number:24886220
Physical property data
1. Properties: translucent white orthorhombic crystals or small pieces.
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 64
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (ºC): Uncertain
10. Autoignition point or ignition temperature (ºC) is uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V /V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Easily soluble in water, slightly soluble in alcohol.
Toxicological data
Acute toxicity: rat oral LD50: >2 gm/kg; rat abdominal LD50: >2 gm/kg; mouse oral LC50: 4 gm/kg.
Ecological data
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None
Compute chemical data
1. Number of hydrogen bond donors: 1
2. Number of hydrogen bond acceptors: 3
3. Number of rotatable chemical bonds: 1
4. Topological molecular polar surface area (TPSA): 60.4
5. Number of heavy atoms: 6
6. Surface charge: 0
7. Complexity :46.1
8. The number of isotope atoms: 0
9. The number of determined atomic stereocenters: 0
10. The number of uncertain atomic stereocenters: 0
11. Determined number of stereocenters of chemical bonds: 0
12. Uncertain number of stereocenters of chemical bonds: 0
13. Number of covalent bond units: 2
Properties and stability
1. Stable under normal temperature and pressure.
2. Incompatible materials: strong oxidants. Easily soluble in water, slightly soluble in alcohol. It has extremely strong reducing properties under high temperature and can make the dyed color disappear, so it is called white lump. It decomposes when exposed to acid, and decomposes at 120°C to produce toxic gases such as formaldehyde and hydrogen sulfide. Anhydrous suspension white blocks are stable but will gradually decompose in humid air. Aqueous solutions begin to decompose above 60°C, and dilute solutions decompose much faster than concentrated solutions.
3., the aqueous solution is relatively stable below 50℃. It decomposes into sulfite at high temperature. It is easy to oxidize and deliquesce when in contact with air. It dissolves in Water is alkaline, relatively stable in neutral or alkaline solutions, extremely unstable in acidic media, and acts as a reducing agent at high temperatures. This product is more stable than insurance powder.
Storage method
Should be stored in a cool, dry warehouse, and the containers must be sealed to prevent moisture and deterioration.
Synthesis method
In the zinc powder sulfur dioxide formaldehyde method, the slurry made of water and zinc powder reacts with sulfur dioxide gas to generate low zinc sulfite, which is then gradually added to the second reactor pre-filled with formaldehyde solution. Under heating and stirring, the slurry is gradually Add zinc powder and react at 95-105°C to generate a suspension of basic zinc bisulfoxylate formaldehyde, which is sent to the third reactor. The crystals are first washed with water, then left to stand, the clear liquid is extracted, and caustic soda solution is added for reaction to generate the second The suspension of sodium bisulfate formaldehyde and zinc hydroxide is filtered, the filtrate is clarified, concentrated, crystallized and pulverized to obtain the finished sodium bisulfite formaldehyde product.

Purpose
1. In the printing and dyeing industry, it is used as a dye discharge agent and reducing agent to produce indigo dyes, vat dyes, etc.
2. Used in synthetic rubber, sugar production and polymerization of vinyl compounds.
3.Used for resist printing of aniline black and soluble vat dyes. Used in food industry, soap industry, rubber synthesis and sugar as bleaching agent

 微信扫一扫打赏
微信扫一扫打赏

