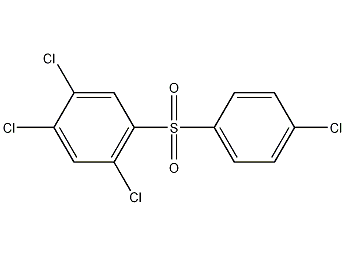
Structural formula
| Business number | 038M |
|---|---|
| Molecular formula | C12H6Cl4O2S |
| Molecular weight | 356.05 |
| label |
2,4,4′,5,tetrachlorodiphenylsulfone, Tidien, The sky and the earth are red, Peaceful earth, Complete retreat, Akaritox, Tedion, Tetradiphon, 2,4,4′,5,Tetrachlorodiphenyl sulfone, Acaricide |
Numbering system
CAS number:116-29-0
MDL number:MFCD00018127
EINECS number:204-134-2
RTECS number:WR5850000
BRN number:2292528
PubChem number:24899294
Physical property data
1. Properties: white crystal, industrial product is yellow to light brown crystalline solid.
2. Density (g/mL, 25℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 145~146
5. Boiling point (ºC, normal pressure): Undetermined p>
6. Boiling point (ºC, KPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): 3.199×10-8
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol ): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (polymer) Log value of the partition coefficient (alcohol/water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V ) Undetermined
19. Solubility: Insoluble in water, slightly soluble in ethanol, soluble in chloroform, acetone, benzene, etc.
Toxicological data
1. Acute toxicity: Mouse oral LD50: 5000mg/kg;Rat acute oral LD50>5000mg/kg
Ecological data
Slightly harmful to water.
Molecular structure data
1. Molar refractive index: 79.58
2. Molar volume (cm3/mol): 225.5
3. Isotonic specific volume (90.2K ): 605.6
4. Surface tension (dyne/cm): 51.9
5. Polarizability (10-24cm3): 31.55
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 42.5
7. Number of heavy atoms: 19
8. Surface charge: 0
9. Complexity: 400
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Chemically stable, it is not easily decomposed by general acid, alkali solutions, high temperatures and ultraviolet rays, and is non-corrosive. Avoid contact with strong oxidizing agents.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. The intermediate trichlorobenzenesulfonyl chloride is obtained by chlorosulfonation of trichlorobenzene, and then condensed with chlorobenzene in the presence of aluminum trichloride to obtain trichlorosulfone. Raw material consumption quota: trichlorobenzene 750kg/t, chlorosulfonic acid 1570kg/t, trichlorobenzenesulfonyl chloride 990kg/t, chlorobenzene 440kg/t, aluminum trichloride 500kg/t.
2. Chlorosulfonation: Add trichlorobenzene dropwise to chlorosulfonic acid, excess chlorosulfonic acid, reaction temperature 80~90°C, chlorosulfonation time 3 hours. The gases released by the reaction are absorbed with water. The generated trichlorobenzenesulfonyl chloride is precipitated by cooling, washed with water (if necessary, a small amount of nitric acid is added to oxidize the low-sulfur compound into trichlorobenzenesulfonyl chloride), and finally dehydrated and dried before being used for condensation.
Condensation Trichlorobenzene sulfonyl chloride and chlorobenzene condense to form trichlorosulfone. The reaction is an electrophilic secondary reaction, using AlCl3 or FeCl3 as catalyst, chlorobenzene is added dropwise, the reaction temperature is 145~155℃, and the reaction time is 1.5~3h. The condensation product is poured into hot water at 90°C, stirred to precipitate trichlorosulfone, washed with water, and dried to obtain the original powder.
Purpose
It has a good effect of killing mite eggs and young mites, with a residual effect period of 40 to 50 days. It can be used to control cotton, citrus, apple and other plants when they first hatch, when their eggs are in full bloom, or when there are fewer adult mites. When used at a content of .05%, it can inhibit egg-laying and infertility of female mites.

 微信扫一扫打赏
微信扫一扫打赏

