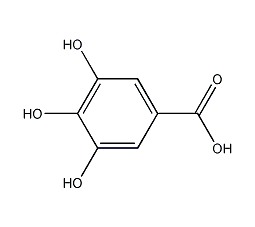
Structural formula
| Business number | 03XZ |
|---|---|
| Molecular formula | C7H6O5 |
| Molecular weight | 170.12 |
| label |
graphite acid, 3,4,5-Trihydroxybenzoic acid anhydrous, oxidised graphite, Gallussaure |
Numbering system
CAS number:149-91-7
MDL number:MFCD00002510
EINECS number:205-749-9
RTECS number:LW7525000
BRN number:2050274
PubChem number:24895304
Physical property data
1. Properties: White or light yellow needle-like crystals or powder
2. Relative density: 1.694
3. Melting point (decomposition, ℃): 253~240
p>
4. Solubility: Soluble in hot water, ether, ethanol, acetone and glycerol, insoluble in cold water, insoluble in benzene and chloroform.
Toxicological data
1. Acute toxicity: rat subcutaneous LDLo: 5 gm/kg;
Mouse intraperitoneal LD50: 4300 mg/kg;
Mouse intravenous LD50: 320 mg/kg kg;
Rabbit oral LD50: 5 gm/kg.
2. Reproductive toxicity: rat subcutaneous TDLo: 5 mg/kg
Ecological data
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 38.82
2. Molar volume (cm3/mol): 97.2
3. Isotonic specific volume (90.2K): 314.4
4. Surface tension (dyne/cm): 109.2
5. Polarizability (10-24cm3): 15.39
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 14
6. Topological molecule polar surface area 98
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 169
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Acidic and astringent.
Storage method
Store sealed in a dry and cool place.
Synthesis method
Made from gallnuts. The main component of gallnuts is tannic acid, which is a hydrolyzable tannin. It can be dissolved in hot water and has an astringent taste. It is an amorphous and complex organic substance. Chinese gallic tannic acid is a complex mixture formed by the combination of gallic acid, double acid and glucose in the form of glycosides or esters. Tannic acid is hydrolyzed under pressure to produce gallic acid and glucose. During hydrolysis, gallic acid ester is first generated, and then digallic acid ester is hydrolyzed into gallic acid. The hydrolysis temperature is 133-135°C and the pressure is 0.18-0.2MPa. Starting from increasing the pressure to 0.18MPa, the reaction was maintained for 2 hours to reach the end point. Compared with normal pressure hydrolysis, sulfuric acid and steam can be saved, and the production capacity can be increased by 25%.
Purpose
1. Gallic acid has many uses in pharmaceuticals, inks, dyes, food, light industry and organic synthesis. Gallic acid and ferric ions form a blue-black precipitate, which is the raw material of blue-black ink; it is also used in tanning industry; it can also be used as a photographic developer. Propyl gallate is an antioxidant and can be used in edible oils to prevent rancidity and deterioration. In medicine, gallic acid is a hemostatic astringent and a mild local irritant.
2. It has antibacterial effect and can treat bacillary dysentery. It has astringent, hemostatic and antidiarrheal effects. Can be used as a preservative. It can prepare pyrogallic acid, drugs, inks, mordant dyes and explosion-proof agents, etc. It is also used as a developer and analytical reagent for the detection of free inorganic acids, dihydroxyacetone, alkaloids and metals.
3. It can be used as a preservative and can be used to prepare mordant dyes and explosion-proof agents.

 微信扫一扫打赏
微信扫一扫打赏

