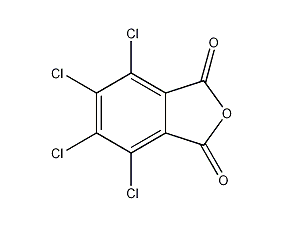
Structural formula
| Business number | 038V |
|---|---|
| Molecular formula | C8Cl4O3 |
| Molecular weight | 285.90 |
| label |
4,5,6,7-tetrachloro-1,3-isobenzofuran, Tetrachlorophthalic anhydride, Tetrachlorophthalic anhydride, Tetrafluorophthalic acid, 4,5,6,7-Tetrachloro-1,3-isobenzofuran, tetrachlorophthalic anhydride, tetrachloro-phthalic anhydride, PTFE o-benzoic acid, plasticizer, flame retardant, Hardener |
Numbering system
CAS number:117-08-8
MDL number:MFCD00005920
EINECS number:204-171-4
RTECS number:TI3450000
BRN number:211560
PubChem number:24848039
Physical property data
1. Properties: colorless needle-like crystals or powder
2. Melting point (ºC): 255℃ (sublimation)
3. Boiling point (ºC, normal pressure): 366℃-371
4. Autoignition point or ignition temperature (ºC): 362
5. Solubility: soluble in dioxane, insoluble in ether, insoluble in cold water.
Toxicological data
1. Acute toxicity: rat inhalation LC: >3600mg/m3/4H; rat oral LD5O: >15800mg/kg; rabbit dermal LD5O: >5g/kg
Ecological data
This substance is seriously harmful to the environment. Do not let this substance enter the environment.
Molecular structure data
1. Molar refractive index: 55.26
2. Molar volume (cm3/mol): 150.3
3. Isotonic specific volume (90.2K ): 431.6
4. Surface tension (dyne/cm): 67.9
5. Polarizability (10-24cm3): 21.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 43.4
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 291
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable at room temperature and pressure, avoid contact with strong oxidants and moisture. Decomposes into tetrachlorophthalic acid in hot water. Odorless and non-hygroscopic.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and water sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
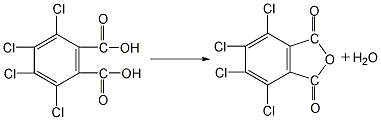
1. Preparation by chlorination of phthalic anhydride. Add phthalic anhydride, 40% fuming sulfuric acid and a small amount of iodine into the reaction kettle, stir and heat to 70°C, add dry chlorine gas, react in the dark for 50 hours, raise the temperature and continue the reaction at 100°C. After 1.5h, add some reaction medium (40% oleum). And raise the temperature to 220°C. After the reaction for a certain period of time, cool down, put the product into a large amount of water, crystallize and precipitate, filter and wash with cold water to obtain the finished product. 2. Preparation by chlorination of phthalic anhydride using hydrosulfonic acid as solvent and Lewis acid such as aluminum trichloride as catalyst. Add a certain amount of chlorosulfonic acid, catalyst and phthalic anhydride into a special chlorination kettle, and then raise the temperature according to a certain program. When the temperature in the reaction kettle rises to 80°C, chlorine gas will be introduced and the solvent and catalyst will be recovered. The distillation temperature shall not exceed 220℃ to avoid product decomposition. After the remaining material is cooled, it is washed with cold water, centrifuged and dehydrated, and dried at 150°C to obtain the finished product. 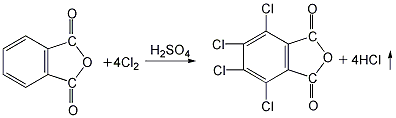 3. Preparation of tetrachlorophthalic acid by dehydration Put it into the reactor and heat it to 98°C to remove one molecule of the two hydroxyl groups in the molecule to obtain the finished product.
3. Preparation of tetrachlorophthalic acid by dehydration Put it into the reactor and heat it to 98°C to remove one molecule of the two hydroxyl groups in the molecule to obtain the finished product.
Purpose
1. Can be used for polyester and epoxy resin, but the effect is not as good as tetrabromophthalic anhydride. Tetrachlorophthalic anhydride is also an intermediate in the organic synthesis of pesticides (rice blast phthalein), dyes, drugs, plasticizers, fire retardant paints, etc. It is used as an intermediate for phthalocyanine green pigments and zanthen series dyes, and is also a raw material for alkyd resins. This product is used as a reactive flame accelerant and curing agent for flame retardant epoxy resin. This product is also an intermediate for organic synthesis of pesticides, dyes, drugs, plasticizers, fire retardant paints, etc.
2. Tetrachlorophthalic anhydride is widely used in flame retardants, pesticides, pigments, coatings and other industries, especially as a reactive flame retardant or flame retardant curing agent for unsaturated polyester and epoxy resins. Not only does the resin have good flame retardancy and mechanical properties, but it also has good high temperature resistance and light radiation stability. A series of flame-retardant plasticizers with excellent comprehensive properties can also be produced.

 微信扫一扫打赏
微信扫一扫打赏

