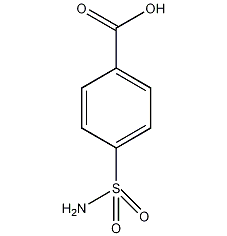
Structural formula
| Business number | 03RY |
|---|---|
| Molecular formula | C7H7NO4S |
| Molecular weight | 201.20 |
| label |
4-Carboxybenzenesulfonamide, Benzoic acid 4-sulfamide, p-Sulfonamidobenzoic acid, p-sulfonylbenzoic acid, aromatic compounds |
Numbering system
CAS number:138-41-0
MDL number:MFCD00007938
EINECS number:205-327-4
RTECS number:DH6820000
BRN number:1875393
PubChem number:24892374
Physical property data
None
Toxicological data
Acute toxicity data :
Rat abdominal cavity LD50:350mg/kg
Mouse abdominal cavityLD50:>1mg/kg
Ecological data
None
Molecular structure data
Molecular property data: 1, Molar refractive index:45.46 2, Molar volume(m3/mol):130.9 3, Isotonic specific volume(90.2K):373.4 4, Surface tension(3.0 dyne/ cm):66.2 5, Polarizability(0.5 10 -24cm3):18.02
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 106
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 284
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
It is obtained by oxidizing p-toluenesulfonamide with potassium dichromate. Another oxidation operation is to react p-toluenesulfonamide with potassium permanganate
Purpose
Used as an organic synthesis intermediate or for drug synthesis.
amily: Arial”>5、 Polarizability(0.5 10-24cm3): 18.02
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 106
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 284
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
It is obtained by oxidizing p-toluenesulfonamide with potassium dichromate. Another oxidation operation is to react p-toluenesulfonamide with potassium permanganate
Purpose
Used as an organic synthesis intermediate or for drug synthesis.

 微信扫一扫打赏
微信扫一扫打赏

